📖 Guidance for Healthcare Employers and Workers on the use of Fluid Resistant Surgical Masks (FRSM) and respirators (FFP3 etc) to comply with the requirements of COSHH (2002) (England) (Updated 2025)
‘FRSMs [Surgical Masks] have never been considered either adequate or suitable equipment for protection against inhalable or respirable risks under COSHH.’
✾
‘Healthcare workers and employers will rightly focus on the safe and effective provision of patient care for patients.
This is the central concern of the Health and Social Care Act, which creates the architecture for IPC [Infection Prevention and Control].
The central aim of this Act is to ensure that the environment is managed effectively to prevent the transmission of infections and that healthcare workers do not inadvertently act as agents for transmission, either because of insufficient hygiene precautions, or because they are themselves infected and could therefore infect others.
Biological hazards can be transmitted via an oral/faecal route, or bloodborne routes, by droplet, fluid splashes, airborne particles or any combination.
COSHH requires all possible routes of exposure to be controlled.
FRSMs [Surgical Masks] have never been considered either adequate or suitable equipment for protection against inhalable or respirable risks under COSHH.
The only respiratory protective equipment [RPE] designated as such for the protection against biological agents is the FFP3 or another respirator type with the same assigned protection factor (APF) (e.g. reusable ‘elastomeric’ respirators, or powered hoods with the appropriate filter).
Failure to comply with COSHH and control standards for RPE is a criminal offence.
This relates to:
(a) the employer’s duty to provide suitable RPE and ensure that it is properly used, inspected and maintained;
and (b) the employee’s duty to wear the RPE as instructed by the employer and to take reasonable care of it.’
✾
‘COSHH and Healthcare Respiratory Protection: Guidance for Healthcare Employers and Workers on the use of Fluid Resistant Surgical Masks (Type IIR) and respirators (FFP3 etc) to comply with the requirements of the Control of Substances Hazardous to Health Regulations (COSHH) (2002) (England) (Updated 2025).’
© 2025 British Occupational Hygiene Society.
[Abridged] ‘COSHH and Healthcare Respiratory Protection’
‘Respiratory infections have long been a recognised hazard of working within healthcare settings.
The principal concern of healthcare legislation and healthcare employers has been the protection of patients who are likely to be vulnerable to such infections, with precautions designed accordingly.’
Page 1
‘Equipment designed to protect the wearer against infection by inhalable pathogens is “respiratory protective equipment” (RPE), a type of personal protective equipment (PPE) designed to reduce (or eliminate) the risk of inhalation of infected particles.
Examples of RPE include FFP3, FFP2 (and N95 [and N99/N100]) respirators which work by the wearer of a respirator drawing in air through filters designed to remove infected particles from the air.’
Page 1
‘If any control, whether through segregation, barrier controls, [or] ventilation, leaves any risk of exposure of an individual worker to a respiratory hazard, then so far as it is reasonably practicable, RPE must be provided and worn in line with health and safety regulations.’
Page 2
‘Training on duties in relation to respiratory protection and the management of RPE has not been widespread through the health service.
There has also been some confusion in Government bodies as to who has regulatory oversight of RPE in the health service as well as the applicable science.
In 2024, the World Health Organisation (WHO), recognising a global need for greater clarity, reviewed the approach to respiratory infection and control.
The new approach recommended by WHO has not been incorporated into UK IPC guidance but is entirely consistent with UK Health and Safety Law.’
Page 3
‘Healthcare workers and employers will rightly focus on the safe and effective provision of patient care for patients.
This is the central concern of the Health and Social Care Act, which creates the architecture for IPC.
The central aim of this Act is to ensure that the environment is managed effectively to prevent the transmission of infections and that healthcare workers do not inadvertently act as agents for transmission, either because of insufficient hygiene precautions, or because they are themselves infected and could therefore infect others.’
Page 3
‘The overriding legal duty is to protect the health and safety of employees, as well as others for whom an undertaking will impact (including patients, service users, contractors and visitors).
Considerations should not be driven by whether someone has a patient-facing or clinical function, is an employee, is in one healthcare setting or another, but should be focused on the risk to that person of exposure to substances hazardous to their health’
Page 4
‘Patients and service users may be vulnerable to biological hazards that would not be a concern for a healthy person exposed to an infectious agent.
For this reason, quite rightly, there is a great emphasis placed on ensuring that healthcare workers do not become the inadvertent agents of the spread of pathogens.’
Page 5
‘Every effort should be made to exclude the pathogen from the place of work.
If this is not possible, engineered controls, not dependent on individuals for their effectiveness, should be deployed, e.g. negative pressure treatment areas or highly effective ventilation.’
Page 6
‘Biological hazards can be transmitted via an oral/faecal route, or bloodborne routes, by droplet, fluid splashes, airborne particles or any combination.
COSHH requires all possible routes of exposure to be controlled.’
Page 6
‘FRSMs [Fluid Repellent Surgical Masks] are, unhelpfully, described as Personal Protective Equipment in the NIPCM.
This is incorrect both in law and in practical terms.
This incorrect designation of FRSMs as PPE or RPE is commonplace within NHS literature and perpetuates a dangerous and illegal inaccuracy.
The only respiratory protective equipment designated as such for the protection against biological agents is the FFP3 or another respirator type with the same assigned protection factor (APF) (e.g. reusable ‘elastomeric’ respirators, or powered hoods with the appropriate filter).’
Page 7
‘FRSMs, as barriers, belong elsewhere in the hierarchy of controls when dealing with respiratory risk, because they do not provide adequate control to prevent the inhalation of infectious particles.
They are not designed to do so and to provide them for that purpose would be in breach of COSHH.’
Page 8
‘[FRSMs’ / Surgical Masks’] effective performance in reducing infection by pathogens such as influenza, even in vitro testing by HSE, will seldom achieve beyond 40%.
Providing an employee with a device that only protects them to that level for a hazardous pathogen would be insufficient and there would be a requirement, in the absence of other highly effective controls, to also provide recognised RPE in order to be legally compliant.’
Page 9
‘FFP3s are made of complex filtering material designed to remove 99% of inhalable and respirable particles.
Of course, they only work as a filter if they are tightly fitted to the face, otherwise they cannot provide that extremely fine filtering as contaminated air will leak into the breathing space through gaps in the face seal.’
Page 9
‘Where there is a risk of exposure of a worker to a significant respiratory hazard which cannot be controlled by other means, including the use of medical devices such as FRSMs, then adequate and suitable RPE must be provided.
Adequate RPE for biological agents is RPE designed to reduce exposure risk to as low as it is reasonably practicable to do so.
Thus, if there is a transmission route involving inhalable respiratory particles such as aerosols, even if a contact transmission route is predominant, then that risk has to be reduced by the use of an FFP3 or equivalent.’
Page 10
‘If RPE fails to achieve the levels of protection it is designed to achieve in healthcare settings, then this is an indication of the failure of the employer to ensure that RPE has been correctly managed to ensure that it achieves its designed protective capability.
This is undoubtedly a breach of Health and Safety law.’
Page 10
‘FRSMs have never been considered either adequate or suitable equipment for protection against inhalable or respirable risks under COSHH.’
Page 10
❦ ‘Guidance for Healthcare Employers and Workers on the use of Fluid Resistant Surgical Masks (Type IIR) and respirators (FFP3 etc) to comply with the requirements of the Control of Substances Hazardous to Health Regulations (COSHH) (2002) (England)’
By The British Occupational Hygiene Society (Updated by BOHS: 2025 / Accessed: 20 Oct 2025)
[Full document] ‘COSHH and Healthcare Respiratory Protection’
Introduction
[Long document ahead: feel free to scroll fast past this...]
Respiratory infections have long been a recognised hazard of working within healthcare settings. The principal concern of healthcare legislation and healthcare employers has been the protection of patients who are likely to be vulnerable to such infections, with precautions designed accordingly.
Prior to the COVID-19 pandemic, regulators regarded instances of harm to healthcare workers from pathogens spread by the respiratory route of transmission (such as influenza, SARS coronaviruses, Respiratory Syncytial Virus [RSV] etc.) as having been relatively rare. This perspective requires revision as a result of the acute and long-term impact of COVID-19 on healthcare workers.
As a consequence of this, healthcare workers and their employers had a great degree of familiarity with medical devices designed to protect patients against respiratory infection by healthcare workers and, indeed, protective equipment designed to protect against contact, ingestion and bloodborne routes. However, awareness of controls to prevent respiratory infection of healthcare workers via the inhalation route has not been as widespread.
Medical devices designed to prevent the wearer from passing on infections to others are typically described as “source control,” because they restrict the source of infection by creating a barrier between the infected wearer and the sterile environment. These devices, like surgical masks, are not personally protective, but interrupt the transmission of infection by reducing the risk that an infected person will pass the infection by creating a barrier to reduce the number of particles, droplets and splashes they might release from their mouth and nose.
The COVID-19 pandemic was, understandably for many, the first time that they were required to deploy or use apparatus designed to protect healthcare workers themselves against inhalation of pathogens. Equipment designed to protect the wearer against infection by inhalable pathogens is “respiratory protective equipment” (RPE), a type of personal protective equipment (PPE) designed to reduce (or eliminate) the risk of inhalation of infected particles. Examples of RPE include FFP3, FFP2 (and N95) respirators which work by the wearer of a respirator drawing in air through filters designed to remove infected particles from the air, and powered filtering respirators which deliver filtered air to the wearer’s breathing zone via a hood.
Understandable confusion has developed between surgical masks (source control) and respirators (wearer personal protection). Both have a role in the reduction of infection prevention and control (IPC), both are worn on the face, both are termed as “PPE” in the National Infection Prevention and Control Manual (NIPCM) and both were recommended by Government and Healthcare Bodies for the control of transmission of COVID-19 at various times and in various places during the pandemic. However, each has a very different function and their use is directed as part of two different systems of legal obligations.
• Surgical masks are devices for use in furtherance of patient safety, as outlined by the Health and Social Care Act 2008. Failure to follow these duties may result in regulatory intervention against a healthcare provider by the Care Quality Commission in England and disciplinary action against a healthcare worker in breach.
• RPE is used to protect workers against infection in furtherance of duties under the Health and Safety at Work Act etc 1974. Breach of duties by an employer is a criminal offence, enforceable by the Health and Safety Executive. It is also a criminal offence for a worker not to comply with these duties and could also result in disciplinary action against a healthcare worker in breach.
The purpose of this guidance note is to ensure that employers and healthcare workers can have a clear understanding of the differences between medical devices used as source control, particularly surgical masks, and RPE, particularly filtering facepiece respirators used as wearer personal protection.
This guidance does not express opinions as to the suitability of each type of device to control specific risks arising from specific pathogens. However, it is a legal requirement in either case that, when determining the suitability of a device to provide control, reference is made to the manufacturer’s instructions. In addition, when using each type of device, scientific and technical guidance produced by the relevant regulator must be followed.
In practice this means that, when using surgical masks as source control (to protect the sterile environment), the Infection Prevention and Control Manual should be followed, as well as any technical and scientific guidance. When using RPE to protect the safety of the wearer, the HSE’s guidance should be followed, as well as any specific technical and scientific guidance.
The IPC regulatory regime is open to clinical interpretation and a wider range of professional discretion using dynamic risk assessment. However, once it has been determined that other methods cannot completely protect a worker against a substance hazardous to health, necessitating the use of RPE, the COSHH Regulations and accompanying official guidance and codes of practice should be followed.
The hierarchy of controls including segregation, ventilation etc is not, however, to be taken as being an order of priority or preference. If any control, whether through segregation, barrier controls, ventilation, leaves any risk of exposure of an individual worker to a respiratory hazard, then so far as it is reasonably practicable, RPE must be provided and worn in line with health and safety regulations. This is not a consideration that is made after the implementation of other controls and where there is evidence of actual control failure, but a decision that needs to be made at the stage of an initial risk assessment and the identification of practicable controls.
Thus, while good general ventilation and source controls such as masks may reduce respirable risk, these measures may not reduce the risk to individuals of inhaling hazardous infectious pathogens, e.g. at close quarters or where the infection is transmitted to some degree by smaller airborne droplets known as aerosols. Such a residual risk of infection, while reduced by other interventions higher up the hierarchy of controls, would require the use of RPE as part of the system of controls to meet the expected threshold under COSHH.
When considering the relative merits of surgical masks and RPE, the primary purpose of either type of device must not be overlooked. Surgical masks aim to contribute to a reduction in overall infections for patients and workers. Failures in adherence and the effectiveness of the device in achieving this is a regulatory performance issue.
Respirators protect the wearer against respiratory exposure to infective agents.
Training on duties in relation to respiratory protection and the management of RPE has not been widespread through the health service. There has also been some confusion in Government bodies as to who has regulatory oversight of RPE in the health service as well as the applicable science.
In 2024, the World Health Organisation (WHO), recognising a global need for greater clarity, reviewed the approach to respiratory infection and control. The new approach recommended by WHO has not been incorporated into UK IPC guidance but is entirely consistent with UK Health and Safety Law.
This guide aims to help employers and worker representatives with a clear statement to outline the differences between medical devices used in IPC (such as the Type IIR mask) on the one hand and RPE on the other. It aims to outline how compliance with IPC measures may not automatically equate to COSHH compliance. Similarly, compliance with COSHH may not necessarily meet IPC standards.
Legal Duties
Healthcare workers and employers will rightly focus on the safe and effective provision of patient care for patients. This is the central concern of the Health and Social Care Act, which creates the architecture for IPC. The central aim of this Act is to ensure that the environment is managed effectively to prevent the transmission of infections and that healthcare workers do not inadvertently act as agents for transmission, either because of insufficient hygiene precautions, or because they are themselves infected and could therefore infect others.
The Act sets out principled requirements for bodies inspectable by the Care Quality Commission Health and Social Care Act 2008: code of practice on the prevention and control of infections and related guidance - GOV.UK.
The Code states:
“However, the code is not mandatory so registered providers do not by law have to comply with the code. A registered provider may be able to demonstrate that it meets the regulations in a different way (equivalent or better) from that described in this document.”
It further states:
“The code does not replace the requirement to comply with any other legislation that applies to health and social care services; for example, the Health and Safety at Work Act 1974 and the Control of Substances Hazardous to Health Regulations 2002.”
All healthcare governance bodies, with responsibilities for guiding employees and patients, as well as employing organisations have legal duties under Health and Safety law. This arises, among other legal duties, in section 2 (1) of the Health and Safety at Work Act.
(1) It shall be the duty of every employer to ensure, so far as is reasonably practicable, the health, safety and welfare at work of all his employees.
Section 3(1) extends the duty to third parties.
Therefore, the following considerations should be kept in mind when formulating guidance, rules, training, protocols and procedures which have an impact on health and safety (as they relate to the use of surgical masks and respirators):
1) The overriding legal duty is to protect the health and safety of employees, as well as others for whom an undertaking will impact (including patients, service users, contractors and visitors);
2) Considerations should not be driven by whether someone has a patient-facing or clinical function, is an employee, is in one healthcare setting or another, but should be focused on the risk to that person of exposure to substances hazardous to their health;
3) Guidance which may be evidence-based in relation to the reduction of infectious risk, which uses administrative methods or medical devices to reduce risk, is a useful element of the hierarchy of controls. However, if there is a residual risk of workers being exposed to hazardous substances, then so far as reasonably practicable, approved RPE must be provided to eliminate that risk.
4) Terminologies, such as “PPE ensemble” and the casual description of medical devices, or fluid resistant surgical masks as “PPE”, should be avoided. Only equipment designed and tested as personal protective equipment, in line with the appropriate standards and which has been determined to adequately control the exposure and which is suitable for the wearer and their work, will be compliant with legal duties.
COSHH vs IPC
Keeping infectious agents out of healthcare areas is difficult. The historic challenges of controlling healthcare acquired infections have led to the current complex infrastructure to help ensure patient safety. Patients and service users may be vulnerable to biological hazards that would not be a concern for a healthy person exposed to an infectious agent.
For this reason, quite rightly, there is a great emphasis placed on ensuring that healthcare workers do not become the inadvertent agents of the spread of pathogens.
The governance and infrastructure of healthcare institutions in the UK is framed by this focus when it comes to IPC. In many contexts, by maintaining good IPC standards, for example sterile environments, good hygiene and the use of bespoke medical devices, healthcare workers will also be protected from exposure.
However, there should not be an automatic assumption that maintaining acceptable and practicable standards of IPC will meet the threshold for protecting workers from exposure as required by COSHH. Equally, standard PPE measures for the control of exposures to workers, such as the use of respirators with exhalation valves, which may compromise the health and safety of others, may need to be re-thought.
The inter-relationship between IPC practices and COSHH practices may seem confusing.
When considered in the context of other concepts, such as the hierarchy of controls, this confusion can escalate further. The use of the same terminology which means different things in two different regulatory systems can be even more confusing.
To explain further, consider this:
1) To prevent the transmission of a pathogen which may be very hazardous to a patient, but not unduly hazardous to a worker, there may be a range of options. It may be accepted that the nature of work that a person does, brings a high probability of infection because of the patients cared for or the role undertaken. If the worker is infected: they may be sent home; deployed away from dealing with certain high-risk patients; be provided with source control equipment (such as surgical masks); or may be immunised so that an infection does not develop in them that can be transmitted further. All of these approaches may be entirely lawful when considering the practicability of preventing exposure to infection.
However, all of these IPC measures still allow the healthcare worker to be infected. The exposure is entirely lawful in IPC terms and can be defensible in COSHH terms if the benefit of completely preventing exposure of the healthcare worker is greatly outweighed by the sacrifice the healthcare provider would have to make to protect the worker.
2) To prevent a pathogen which is directly hazardous to the worker as well as to patients, a different approach may be required. If exposing the worker to a pathogen may have serious and irreversible impact on their health, then the options outlined above would not be lawful.
Every effort should be made to exclude the pathogen from the place of work. If this is not possible, engineered controls, not dependent on individuals for their effectiveness, should be deployed, e.g. negative pressure treatment areas or highly effective ventilation. It would be relatively unusual for a designed solution such as this to work on its own, since compliance by healthcare workers with protocols, such as who is allowed into such a controlled area, need to be in place.
These “administrative controls” rely on compliance and people management.
Finally, however, there will be circumstances where a worker cannot benefit from the protection of the engineered control because, for example, they are providing close quarter care. In these instances, rather than controlling the environment or access to the environment, the focus on controlling the risk must be on protecting the person.
PPE is highly dependent on its correct selection, its correct use and management.
Importantly, the PPE must be suitable to meet the needs of the person, e.g. a person’s physical characteristics such as size and shape (also face size and shape with regard to face seal of FFPs) and the type of work that they do. The suitability of PPE requires a thorough risk assessment (as outlined in HSE guidance, for example), careful consideration, planning and analysis. What works for one person may not work for another. As well as being suitable, PPE needs to adequately protect the wearer so far as is reasonably practicable from the risk of hazardous exposure. This means that the device itself should be designed to be capable of providing a specified degree of protection (as defined in applicable UK designated standards).
It is therefore paramount that all the factors of how PPE may be compromised are considered and appropriately managed. In some ways, this is common sense. A firefighter may attend a fire and may be exposed to flames, fumes, oxygen deprivation, toxic dust, falling debris, heat exhaustion and a range of other risks.
When determining the appropriate PPE, all the potential risks need to be protected against so far as reasonably practicable. If the respirator protects against toxic dust but does not protect against oxygen deprivation, the hazard is not controlled.
The same applies to the control of infectious pathogens. Biological hazards can be transmitted via an oral/faecal route, or bloodborne routes, by droplet, fluid splashes, airborne particles or any combination.
COSHH requires all possible routes of exposure to be controlled. Thus, for infectious diseases, all possible routes of transmission need to be considered unless the level of risk being protected against is marginal compared to the sacrifice required to achieve adequate control. Thus, while a face visor may protect the mucosa from splashes and therefore control a deposition route to infection, it will not control the risk of inhalation of airborne pathogens which may cause infection.
Even if the predominant route of infection is by fluid splashes, if there is another transmission route (e.g. inhalation) and the pathogen is hazardous to health via inhalation, then infection via the inhalation route must also be adequately controlled. That is the legal requirement of COSHH. By contrast, from an IPC point of view, it may be viewed that infection transmission can be most effectively reduced by focusing on the predominant mode of transmission and using other measures, such as the removal of infected staff from the roster.
These differing approaches represent a fundamental difference in the tolerance of risk of infection of individual healthcare workers. The former focuses on the reduction of the risk of exposure for each worker so far as reasonably practicable, whereas the latter looks at the impact in overall infection transmission within the healthcare setting, often with a priority on the extent of patient risk, balanced against operational delivery.
FRSM vs FFP3
The COVID-19 pandemic and the COVID-19 Inquiry has brought into sharp contrast fundamental misunderstandings about IPC measures and COSHH measures. This is typified by ongoing debates about the relative roles of Type IIR Fluid Resistant Surgical Masks (FRSMs) and Filtering Facepiece Respirators - level 3 (FFP3s). Both devices have roles within both the achievement of IPC and COSHH outcomes, but in quite different ways.
Sometimes the debate is unhelpfully abbreviated to “masks” vs “respirators”. Even this simplification is fraught with danger. Conflating all masks together (whether or not they are fluid resistant) would be problematic. Similarly, conflating FFP1 (low level protection), FFP2/N95 (higher level protection) and FFP3 (or N99, the US equivalent of FFP3) is not helpful. Each provides a different level of filtration and consequent protection.
In WHO documentation, NHS research material and submissions to the COVID-19 Inquiry, evidence about the effectiveness of masks vs respirators which conflates these devices and equipment has caused confusion which undermines legal compliance.
FRSMs are, unhelpfully, described as Personal Protective Equipment in the NIPCM. This is incorrect both in law and in practical terms. This incorrect designation of FRSMs as PPE or RPE is commonplace within NHS literature and perpetuates a dangerous and illegal inaccuracy.
The only respiratory protective equipment designated as such for the protection against biological agents is the FFP3 or another respirator type with the same assigned protection factor (APF) (e.g. reusable ‘elastomeric’ respirators or powered hoods with the appropriate filter).
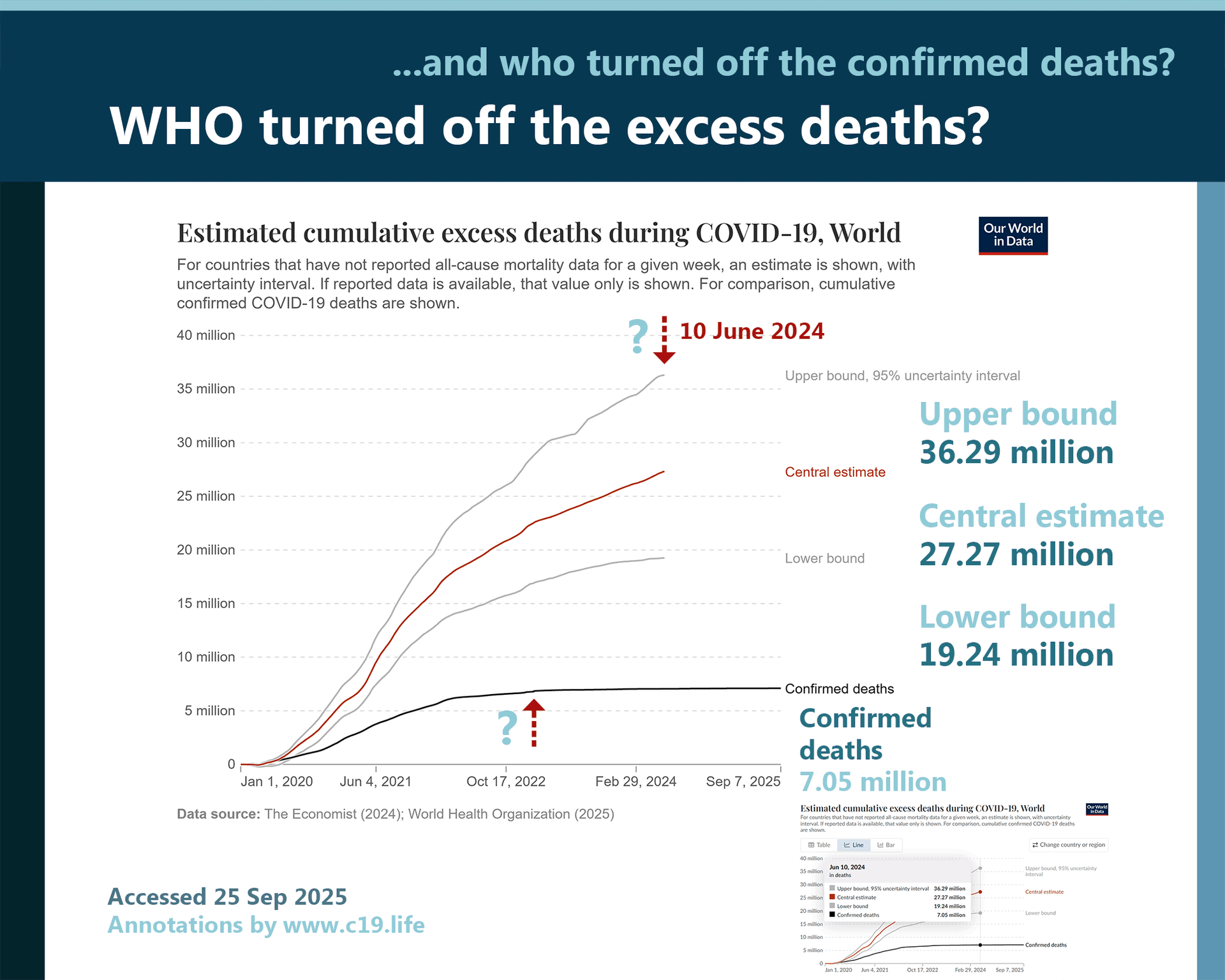
This is because, while both are worn about the face and both have a role in the prevention of infection, they are very different things. In very simple terms, these are the distinctions.
• FFP3s are designed and tested to filter respirable and inhaled particles (including viral and other pathogens). The whole device aims to protect the wearer from anything but the smallest level of exposure, provided it is selected and used correctly.
• FRSMs are designed and tested to create a barrier to exhaled bacteria in droplets and to be made of material which resists pressurised artificial blood. The effect is to reduce the risk of the wearer’s exhaled breath contaminating a sterile environment with bacteria. It also creates a barrier for the wearer against splash contamination by liquids around the nose and mouth, if worn correctly.
(From a technical and legal perspective, the differences are summarised below in Annex I.)
The differences between the underpinning design and operation of FRSM and FFP3 (a barrier and a filter; exhalation and inhalation; droplets containing bacteria and respirable viral particles such as aerosols; protecting others and protecting the wearer) must make it clear that these are two very different types of devices designed for two very different purposes.
In terms of IPC, the premise of an FRSM is that the wearer may be infected with a pathogen transmitted by larger droplets and that the barrier can protect against those droplets having an uninterrupted trajectory into a sterile environment or onto a patient.
They may also present a barrier to splashes and droplets that have a trajectory towards the wearer’s mouth and nose. In that respect, they operate almost the same as a visor barrier or screen. They can provide a vital break in human-human transmission probability, especially where there is a significant role for pathogen transmission by direct deposition of fluids in splashes or droplets.
This is a barrier method of protection, but anything that can circumvent the barrier, such as a particle suspended in the air, can compromise its role as a protective device. Inhaled respirable particles are therefore inherently capable of getting drawn in through the gaps between the mask and the wearer.
FRSMs are not tightly fitted to the face (and are not designed to be) because the fluid resistant material would also make breathing very difficult. The reason for the general requirement of fit testing of RPE is that leakages due to poor seals defeat the very fine filtration design.
FRSMs, as barriers, belong elsewhere in the hierarchy of controls when dealing with respiratory risk, because they do not provide adequate control to prevent the inhalation of infectious particles. They are not designed to do so and to provide them for that purpose would be in breach of COSHH. That does not mean that high mask adherence cannot reduce the risk of transmission of a pathogen which has a deposition transmission route.
However, their effective performance in reducing infection by pathogens such as influenza, even in vitro testing by HSE, will seldom achieve beyond 40%. Providing an employee with a device that only protects them to that level for a hazardous pathogen would be insufficient and there would be a requirement, in the absence of other highly effective controls, to also provide recognised RPE in order to be legally compliant.
FFP3s are made of complex filtering material designed to remove 99% of inhalable and respirable particles. Of course, they only work as a filter if they are tightly fitted to the face, otherwise they cannot provide that extremely fine filtering as contaminated air will leak into the breathing space through gaps in the face seal.
Many FFP3s are fitted with exhalation valves. This makes them largely unsuited to protecting the sterile environment against the risks posed by the wearer if they are infected with a pathogen which could be transmitted by fluids, direct deposition or condensates of respirable particles. Therefore, while valved FFPs protect the wearer from respiratory risk, they may not provide an effective IPC control, if the wearer is infected. Not all FFP3s will meet the impermeability standards for Type IIR FRSMs either, potentially presenting a risk for a fluid-based infection. If there is a risk of wicking of infectious fluid, then an FFP respirator with fluid resistant properties would be appropriate.
The very limited availability of unvalved FFP3 respirators, with fluid resistant properties, capable of achieving an effective fit to faces of healthcare workers during the pandemic reflected a failure to have a secure supply or stockpile of adequate (i.e. designed to achieve the right level of protection) and suitable (i.e. fits the person and works in the context of their activity) RPE. In the context of the pandemic, this lack of availability meant that it may not have in all circumstances been reasonably practicable to meet the standards expected in COSHH while those shortages persisted.
FFP3 respirators with certified fluid resistance (Type IIR), which are designed in vitro to filter 99% of respirable particles, were certified as an alternative to FRSMs to provide IPC control in the same way as FRSMs.
The preponderance of citations in NHS literature (as to the relative effectiveness of masks against respirators in the control of infection) are derived from overseas studies and almost exclusively consider comparisons with N95 filtering facepieces. N95 respirators are equivalent to FFP2 respirators in the UK, which would not be deemed to provide adequate control of respirable particles under UK law.
Clinical studies almost never record the extent to which face fit requirement for N95s has been implemented, meaning the deployment of respirators may not meet the UK’s standards for testing suitability of the equipment to the wearer.
The apparent lack of technical understanding of these fundamental RPE principles by reviewing authors means studies such as The role of respirators and surgical masks in mitigating the transmission of SARS-CoV-2 in healthcare settings need to be read with caution, especially when considering the appropriateness of equipment for the purposes of respiratory protection.
These studies have been cited by bodies such as the UK Health Security Agency to provide only limited evidence that N95s were more effective in controlling infection transmission and/or wearer protection. However, few (if any) conclusions can be drawn from them about FFP3 performance within UK healthcare settings.
In determining the question of duties, the matter is clearer. Where there is a risk of exposure of a worker to a significant respiratory hazard which cannot be controlled by other means, including the use of medical devices such as FRSMs, then adequate and suitable RPE must be provided.
Adequate RPE for biological agents is RPE designed to reduce exposure risk to as low as it is reasonably practicable to do so. Thus, if there is a transmission route involving inhalable respiratory particles such as aerosols, even if a contact transmission route is predominant, then that risk has to be reduced by the use of an FFP3 or equivalent.
That is one leg of the legal duty on employers.
However, the RPE also needs to be selected so as to be capable of delivering the designed protection, i.e. that it is suitable. This means that the device fits the intended wearer, and that it can be worn without disturbance in the working conditions the wearer is likely to encounter. That is the second leg of the duty of the employers.
Thus, if an employer provides RPE but it does not fit (the intended wearer(s)) or is not appropriate for the working conditions within which it is used, the employer cannot abandon the use of RPE in favour of another control, such as barrier, if it is known that it would not provide as effective a control as suitable RPE.
In order for RPE to become licensed for use, it needs to meet a range of stringent tests, which consider and address the potential for failure in a range of workplace environments.
If RPE fails to achieve the levels of protection it is designed to achieve in healthcare settings, then this is an indication of the failure of the employer to ensure that RPE has been correctly managed to ensure that it achieves its designed protective capability.
‘If RPE fails to achieve the levels of protection it is designed to achieve in healthcare settings, then this is an indication of the failure of the employer to ensure that RPE has been correctly managed to ensure that it achieves its designed protective capability.
This is undoubtedly a breach of Health and Safety law.’
This is undoubtedly a breach of Health and Safety law. Such a breach must be remedied by finding adequate and suitable alternative RPE with the required Assigned Protection Factor such as powered filtering respirators (as well as instituting other measures from the hierarchy of controls to reduce residual risk). Such other measures may include using barrier (engineering) controls, such as FRSMs. However, FRSMs have never been considered either adequate or suitable equipment for protection against inhalable or respirable risks under COSHH.
[End of document]
‘FRSMs have never been considered either adequate or suitable equipment for protection against inhalable or respirable risks under COSHH.’
✾
📖 (Accessed: 20 Oct 2025 ~ British Occupational Hygiene Society / Occupational & Environmental Medicine) COSHH and Healthcare Respiratory Protection: Guidance for Healthcare Employers and Workers on the use of Fluid Resistant Surgical Masks (Type IIR) and respirators (FFP3 etc) to comply with the requirements of the Control of Substances Hazardous to Health Regulations (COSHH) (2002) (England) (Updated 2025) ➤ [PDF]
© 2025 British Occupational Hygiene Society.
✾
✾
More... On Healthcare Workers




Solutions in... NHS Estates Bulletins


More on... COSHH

From... The UKHSA
“How does Covid being a notifiable disease interact with the UK government discouraging testing?
How does this work? How does it work with NHS Trusts telling their staff not to test?”
Tern, a Priest (28 Aug 2023)
✾


✾
“If we didn’t do any testing, we would have very few cases.”
Donald John Trump (15 May 2020)
✾
More... Healthcare Worker insights