☰ For Medics ~ 16 studies & articles on... SARS-CoV-2 (COVID-19) and on-going mitochondrial dysfunction
‘... SARS-CoV-2 infection causes prolonged disruptions in mitochondrial function, significantly altering cellular energy metabolism.’
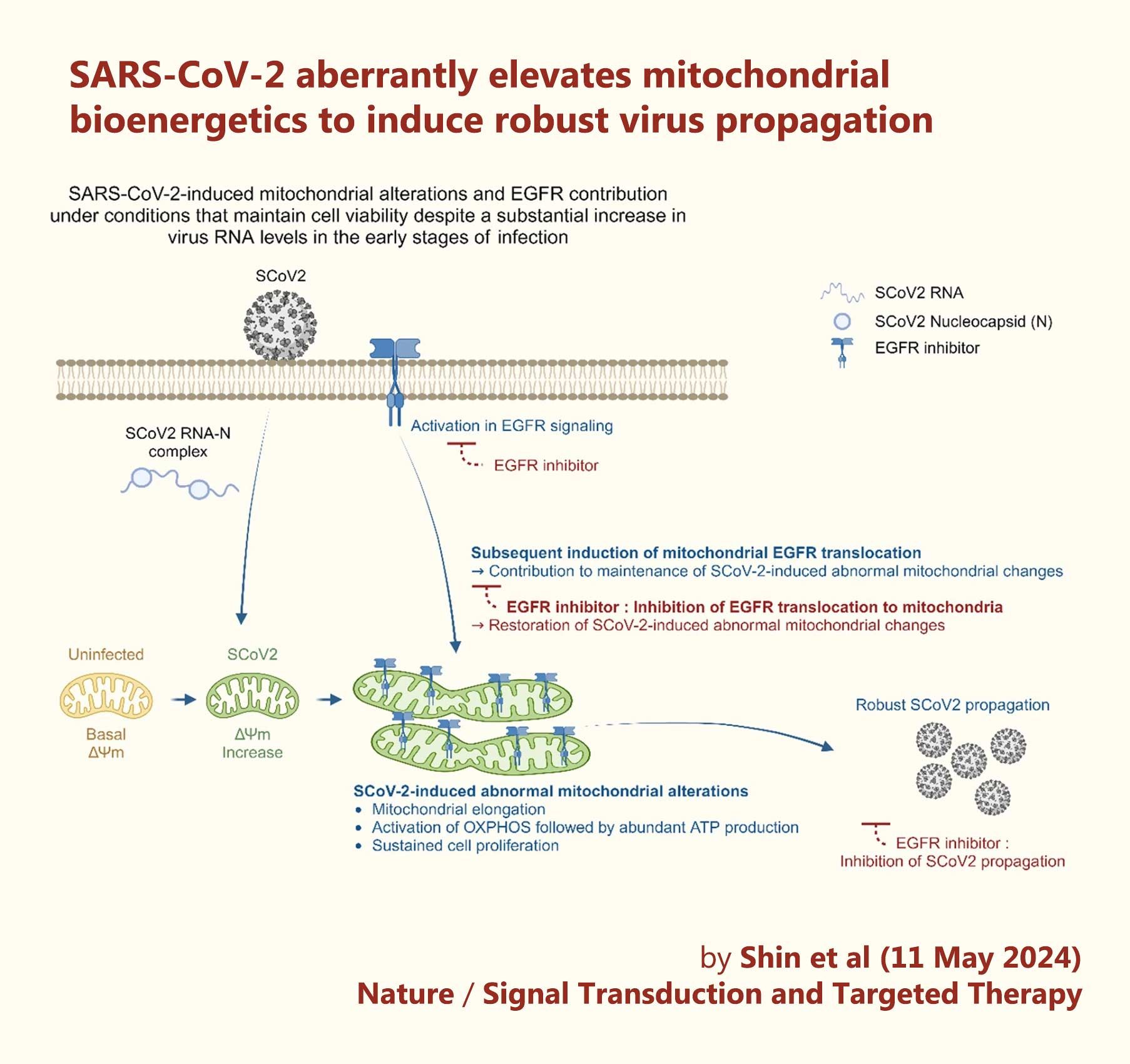
‘A schematic diagram showing the significant contribution of SCoV2-induced altered mitochondrial dynamics and mitochondrial EGFR translocation in sustaining viral propagation.
First, SCoV2 RNA and nucleocapsid complex increase ΔΨm during the early stages of SCoV2 infection. This alteration subsequently promotes mitochondrial elongation. SCoV2 also activates the mitochondrial OXPHOS process, thereby promoting ATP production.
Second, SCoV2 activates EGFR-mediated cell survival signalling and subsequently promotes mitochondrial EGFR internalisation, which contributes to the maintenance of abnormal mitochondrial bioenergetics.
These alterations are physiologically relevant to the maintenance of homoeostasis of SCoV2-infected cells and robust SCoV2 propagation.’
📖 (11 May 2024 ~ Nature: Signal Transduction and Targeted Therapy) SARS-CoV-2 aberrantly elevates mitochondrial bioenergetics to induce robust virus propagation ➤
© 2024 Shin et al / Nature.
❦ On mitochondria and dysfunction
❦ What are mitochondria?
- Mitochondria are small organelles found in nearly all human cells, and are vital to survival.
- They are often referred to as the “powerhouses of the cell” because they generate most of the energy needed for cellular functions by producing adenosine triphosphate (ATP).
- Mitochondria also play roles in cell-signalling, cell growth, and cell death (apoptosis).
❦ Mitochondrial dysfunction and disease
- When mitochondria stop functioning, the cell they are in is starved of energy. Depending on the type of cell, symptoms can vary widely.
- As a general rule, cells that need the largest amounts of energy – such as heart muscle cells and nerves – are affected the most by faulty mitochondria.
- Although symptoms of a mitochondrial disease vary greatly, they might include:
- loss of muscle co-ordination and weakness
- problems with vision or hearing
- heart, liver, or kidney disease
- gastrointestinal problems
- neurological problems, including dementia.
❦ Notes
📖 (12 Aug 2025 ~ Annals of Medicine) Mitochondrial function is impaired in long COVID patients ➤
‘Fatigue, reduced exercise tolerance and hyperlactataemia on minimal exertion [in ‘Long COVID’/PASC cases] have led to the suggestion of a bioenergetic defect.
In conclusion, this study identifies mitochondrial function abnormalities in the PBMCs of Long COVID/PASC cases which are distinct from the mitochondrial phenotype of acute SARS-CoV-2 infection.
Our findings are consistent with the hypothesis that there is an abnormality of complex V function which is evidence of bioenergetic inefficiency.
The results suggest that PBMC mitochondrial function might be a future biomarker of Long COVID/PASC.’
© 2025 Macnaughtan et al / Annals of Medicine.
📖 (30 Jul 2025 ~ Mitochondrion) Peripheral immune progression to long COVID is associated with mitochondrial gene transcription: A meta-analysis ➤
‘We conducted one of the most comprehensive meta-analyses to date of all quality bulk RNA-seq studies worldwide from the COVID-19 pandemic and show significant mitochondrial transcript changes in the peripheral immune system of people with Long COVID, with unexpectedly low levels of intracellular viral RNA in Long COVID.
This extensive analysis, which includes 26 studies and 1,272 individuals, shows that mononuclear cells, PBMC, and granulocytes from Long COVID patients exhibit significant alterations in mitochondrial genes and related processes.’
© 2025 Maison et al / Mitochondrion.
📖 (12 Jul 2025 ~ Molecular Psychiatry) Brain and muscle chemistry in myalgic encephalitis/chronic fatigue syndrome (ME/CFS) and long COVID: a 7T magnetic resonance spectroscopy study ➤
‘An observation of potential importance for future research and treatment development is that the ME/CFS and long COVID groups tested in this study differed in terms of their brain neurochemistry measured by MRS.
It is therefore possible that the underlying neurobiological mechanisms, while leading to similar clinical presentation of fatigue and brain fog, may differ between these groups.
While this needs to be verified with future research, an important implication is that patients with ME/CFS and those with fatigue in the course of long COVID should not be studied as a single group, at least until the mechanisms are better understood.’
© 2025 Godlewska et al / Molecular Psychiatry.
📖 (8 Jul 2025 ~ PNAS) Oxidative stress is a shared characteristic of ME/CFS and Long COVID ➤
‘More than 65 million individuals worldwide are estimated to have Long COVID (LC), wherein individuals after infection report persistent fatigue, post-exertional malaise (PEM), and other symptoms resembling myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
With no clinically approved treatments or diagnostic markers for these conditions, there is an urgent need to define the molecular underpinnings.
By studying bioenergetic characteristics of immune cells in healthy controls, ME/CFS, and LC donors, we find lymphocytes from ME/CFS and LC donors exhibit elevated oxidative stress.
Due to excess oxidative stress and consequent mitochondrial damage, ME/CFS and LC donor lymphocytes consume excess host energy, contributing to debilitating fatigue and other sequelae.’
© 2025 Shankar et al / PNAS.
📖 (9 May 2025 ~ International Journal of Molecular Sciences) Persistent Monocytic Bioenergetic Impairment and Mitochondrial DNA Damage in PASC Patients with Cardiovascular Complications ➤
‘This study identifies persistent mitochondrial dysfunction in long COVID monocytes as a critical driver of cardiovascular complications in PASC.
Key defects – bioenergetic failure, impaired stress adaptation and mtDNA damage – correlate with clinical symptoms like heart failure and exercise intolerance.’
© 2025 Semo et al / International Journal of Molecular Sciences.
📖 (4 Nov 2024 ~ GeroScience) Novel biomarkers of mitochondrial dysfunction in Long COVID patients ➤
‘COVID-19 can lead to severe acute respiratory syndrome, and while most individuals recover within weeks, approximately 30–40% experience persistent symptoms collectively known as Long COVID, post-COVID-19 syndrome, or post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC).
According to recent studies, SARS-CoV-2 infection causes prolonged disruptions in mitochondrial function, significantly altering cellular energy metabolism.
Our research employed transmission electron microscopy to reveal distinct mitochondrial structural abnormalities in Long COVID patients, notably including significant swelling, disrupted cristae, and an overall irregular morphology, which collectively indicates severe mitochondrial distress.’
© 2024 Szögi et al / GeroScience.
📖 (30 Apr 2024 ~ Pharmacological Research) SARS-CoV-2 mitochondrial metabolic and epigenomic reprogramming in COVID-19 ➤
‘To determine the effects of SARS-CoV-2 infection on cellular metabolism, we conducted an exhaustive survey of the cellular metabolic pathways modulated by SARS-CoV-2 infection and confirmed their importance for SARS-CoV-2 propagation by cataloging the effects of specific pathway inhibitors.
This revealed that SARS-CoV-2 strongly inhibits mitochondrial oxidative phosphorylation (OXPHOS) resulting in increased mitochondrial reactive oxygen species (mROS) production.
The elevated mROS stabilizes HIF-1α which redirects carbon molecules from mitochondrial oxidation through glycolysis and the pentose phosphate pathway (PPP) to provide substrates for viral biogenesis. mROS also induces the release of mitochondrial DNA (mtDNA) which activates innate immunity.
The restructuring of cellular energy metabolism is mediated in part by SARS-CoV-2 Orf8 and Orf10 whose expression restructures nuclear DNA (nDNA) and mtDNA OXPHOS gene expression.
These viral proteins likely alter the epigenome, either by directly altering histone modifications or by modulating mitochondrial metabolite substrates of epigenome modification enzymes, potentially silencing OXPHOS gene expression and contributing to long-COVID.’
© 2024 Guarnieri et al / Pharmacological Research.
📖 (25 Aug 2023 ~ Frontiers in Cellular Infectious Microbiology) Host mitochondria: more than an organelle in SARS-CoV-2 infection ➤
‘The severe form of COVID-19 is often marked by an altered immune response and cytokine storm. Advanced age, age-related and underlying diseases, including metabolic syndromes, appear to contribute to increased COVID-19 severity and mortality suggesting a role for mitochondria in disease pathogenesis.
Furthermore, since the immune system is associated with mitochondria and its damage-related molecular patterns (mtDAMPs), the host mitochondrial system may play an important role during viral infections.
Viruses have evolved to modulate the immune system and mitochondrial function for survival and proliferation, which in turn could lead to cellular stress and contribute to disease progression.
Recent studies have focused on the possible roles of mitochondria in SARS-CoV-2 infection. It has been suggested that mitochondrial hijacking by SARS-CoV-2 could be a key factor in COVID-19 pathogenesis.
In this review, we discuss the roles of mitochondria in viral infections, including SARS-CoV-2 infection, based on past and present knowledge.’
© 2023 Shoraka et al / Frontiers in Cellular Infectious Microbiology.
📖 (9 Aug 2023 ~ Science Translational Medicine) Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts ➤
‘SARS-CoV-2 needs host cells to generate molecules for viral replication and propagation.
Guarnieri et al now show that the virus is able to block expression of both nuclear-encoded and mitochondrial-encoded mitochondrial genes, resulting in impaired host mitochondrial function.
They analyzed human nasopharyngeal samples and autopsy tissues from patients with COVID-19 and tissues from hamsters and mice infected with SARS-CoV-2.
Host cells attempt to compensate by activating innate immune defenses and mitochondrial gene expression, but chronically impaired mitochondrial function ultimately may result in serious COVID-19 sequelae such as organ failure.’
© 2023 Guarnieri et al / Science Translational Medicine.
📖 (21 Jan 2022 ~ iScience) SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics ➤
‘Here, we conducted transcriptomic analysis of human PBMCs, identified significant changes in mitochondrial, ion channel, and protein quality-control gene products.
SARS-CoV-2 proteins selectively target cellular organelle compartments, including the endoplasmic reticulum and mitochondria.
M-protein, NSP6, ORF3A, ORF9C, and ORF10 bind to mitochondrial PTP complex components cyclophilin D, SPG-7, ANT, ATP synthase, and a previously undescribed CCDC58 (coiled-coil domain containing protein 58).
Knockdown of CCDC58 or mPTP blocker cyclosporin A pretreatment enhances mitochondrial Ca2+ retention capacity and bioenergetics.
SARS-CoV-2 infection exacerbates cardiomyocyte autophagy and promotes cell death that was suppressed by cyclosporin A treatment.
Our findings reveal that SARS-CoV-2 viral proteins suppress cardiomyocyte mitochondrial function that disrupts cardiomyocyte Ca2+ cycling and cell viability.’
© 2022 Ramachandran et al / iScience.
📖 (17 Dec 2021 ~ iScience) Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis ➤
‘Here, we analyzed available bulk RNA-seq datasets from COVID-19 patients and corresponding healthy controls (three blood datasets, N = 48 healthy, 119 patients; two respiratory tract datasets, N = 157 healthy, 524 patients).
We found significantly reduced mtDNA gene expression in blood, but not in respiratory tract samples from patients.
Next, analysis of eight single-cells RNA-seq datasets from peripheral blood mononuclear cells, nasopharyngeal samples, and Bronchoalveolar lavage fluid (N = 1,192,243 cells), revealed significantly reduced mtDNA gene expression especially in immune system cells from patients.
This is associated with elevated expression of nuclear DNA-encoded OXPHOS subunits, suggesting compromised mitochondrial-nuclear co-regulation.
This, together with elevated expression of ROS-response genes and glycolysis enzymes in patients, suggest rewiring toward glycolysis, thus generating beneficial conditions for SARS-CoV-2 replication.
Our findings underline the centrality of mitochondrial dysfunction in COVID-19.’
© 2021 Medini et al / iScience.
📖 (15 Mar 2021 ~ Nature Communications) SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication ➤
‘The recently identified Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the cause of the COVID-19 pandemic.
How this novel beta-coronavirus virus, and coronaviruses more generally, alter cellular metabolism to support massive production of ~30 kB viral genomes and subgenomic viral RNAs remains largely unknown.
To gain insights, transcriptional and metabolomic analyses are performed 8 hours after SARS-CoV-2 infection, an early timepoint where the viral lifecycle is completed but prior to overt effects on host cell growth or survival.
Here, we show that SARS-CoV-2 remodels host folate and one-carbon metabolism at the post-transcriptional level to support de novo purine synthesis, bypassing viral shutoff of host translation.
Intracellular glucose and folate are depleted in SARS-CoV-2-infected cells, and viral replication is exquisitely sensitive to inhibitors of folate and one-carbon metabolism, notably methotrexate.
Host metabolism targeted therapy could add to the armamentarium against future coronavirus outbreaks.’
© 2021 Zhang et al / Nature Communications.
📖 (6 Jan 2021 ~ American Journal of Physiology Cell Physiology) Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19 ➤
‘Patients with metabolic syndrome suffer from severe complications and a higher mortality rate due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
We recently proposed that SARS-CoV-2 can hijack host mitochondrial function and manipulate metabolic pathways for their own advantage.
The aim of the current study was to investigate functional mitochondrial changes in live peripheral blood mononuclear cells (PBMCs) from patients with COVID-19 and to decipher the pathways of substrate utilization in these cells and corresponding changes in the inflammatory pathways.
We demonstrate mitochondrial dysfunction, metabolic alterations with an increase in glycolysis, and high levels of mitokine in PBMCs from patients with COVID-19.
Interestingly, we found that levels of fibroblast growth factor 21 mitokine correlate with COVID-19 disease severity and mortality.
These data suggest that patients with COVID-19 have a compromised mitochondrial function and an energy deficit that is compensated by a metabolic switch to glycolysis.
This metabolic manipulation by SARS-CoV-2 triggers an enhanced inflammatory response that contributes to the severity of symptoms in COVID-19. Targeting mitochondrial metabolic pathway(s) can help define novel strategies for COVID-19.’
© 2021 Ajaz et al / American Journal of Physiology Cell Physiology.
📖 (20 Jul 2020 ~ American Journal of Physiology Cell Physiology) Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis ➤
‘Based on available data for the SARS-CoV-1 virus, we suggest how CoV-2 localization of RNA transcripts in mitochondria hijacks the host cell’s mitochondrial function to viral advantage.
Besides viral RNA transcripts, RNA also localizes to mitochondria.
SARS-CoV-2 may manipulate mitochondrial function indirectly, first by ACE2 regulation of mitochondrial function, and once it enters the host cell, open-reading frames (ORFs) such as ORF-9b can directly manipulate mitochondrial function to evade host cell immunity and facilitate virus replication and COVID-19 disease.
Manipulations of host mitochondria by viral ORFs can release mitochondrial DNA (mtDNA) in the cytoplasm and activate mtDNA-induced inflammasome and suppress innate and adaptive immunity.
We argue that a decline in ACE2 function in aged individuals, coupled with the age-associated decline in mitochondrial functions resulting in chronic metabolic disorders like diabetes or cancer, may make the host more vulnerable to infection and health complications to mortality.
These observations suggest that distinct localization of viral RNA and proteins in mitochondria must play essential roles in SARS-CoV-2 pathogenesis.
Understanding the mechanisms underlying virus communication with host mitochondria may provide critical insights into COVID-19 pathologies. An investigation into the SARS-CoV-2 hijacking of mitochondria should lead to novel approaches to prevent and treat COVID-19.’
© 2020 Singh et al / American Journal of Physiology Cell Physiology.
📖 (17 Jul 2020 ~ Cell Metabolism) Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/Glycolysis-Dependent Axis ➤
‘COVID-19 can result in severe lung injury.
It remained to be determined why diabetic individuals with uncontrolled glucose levels are more prone to develop the severe form of COVID-19.
The molecular mechanism underlying SARS-CoV-2 infection and what determines the onset of the cytokine storm found in severe COVID-19 patients are unknown.
Monocytes and macrophages are the most enriched immune cell types in the lungs of COVID-19 patients and appear to have a central role in the pathogenicity of the disease.
These cells adapt their metabolism upon infection and become highly glycolytic, which facilitates SARS-CoV-2 replication.
The infection triggers mitochondrial ROS production, which induces stabilization of hypoxia-inducible factor-1α (HIF-1α) and consequently promotes glycolysis.
HIF-1α-induced changes in monocyte metabolism by SARS-CoV-2 infection directly inhibit T cell response and reduce epithelial cell survival.
Targeting HIF-1ɑ may have great therapeutic potential for the development of novel drugs to treat COVID-19.’
© 2020 Codo et al / Cell Metabolism.
‘Dear Secretary Robert Kennedy Jr*, with your expressed concern about many children now showing signs of mitochondrial dysfunction, here is evidence that a pathogen we continue to allow to circulate without mitigations is known to cause mitochondrial dysfunction.’
by Dr. Andrew Urquhart, 29 Aug 2025 (Twitter / X ➤)
* United States of America’s Secretary of Health and Human Services (HHS) (Feb 13, 2025 –)
❦ Studies presented on this page collated by Dr. Andrew Urquhart (PhD). Excerpts collated by C19.Life.
More... ☰ Lists



More by... C19.Life