☰ The beating heart... 70 studies & articles on SARS-CoV-2 and the cardiovascular system (2020–2023/2024)
‘But even people who had not been hospitalized had increased risks of many conditions, ranging from an 8% increase in the rate of heart attacks to a 247% increase in the rate of heart inflammation.’
Nature (2 Aug 2022)
‘Either symptomatic or asymptomatic SARS-CoV-2 infection is associated with increased risk of late cardiovascular outcomes and has causal effect on all-cause mortality in a late post-COVID-19 period.’
The American Journal of Cardiology (15 Sep 2023)
📖 (2 Mar 2022 ~ JAMA) ‘The COVID Heart – One Year After SARS-CoV-2 Infection, Patients Have an Array of Increased Cardiovascular Risks’. ➲
‘Patients with COVID-19 were at increased risk of a broad range of cardiovascular disorders including cerebrovascular disorders, dysrhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure and thromboembolic disease.’
© 2022 Jennifer Abbasi / JAMA.
📖 (19 Mar 2024 ~ European Society of Cardiology: Cardiovascular Research) ‘Persistent increase of cardiovascular and cerebrovascular events in COVID-19 patients: a 3-year population-based analysis’. ➲
‘The increase of cardiovascular risk associated with COVID-19 might be extended for years and not limited to the acute phase of the infection.’
© 2024 Battistoni & Volpe et al / European Society of Cardiology: Cardiovascular Research.
📖 (14 Mar 2022 ~ Johns Hopkins) ‘COVID and the Heart: It Spares No-One’. ➲
‘New evidence has revealed that anyone infected with COVID is at higher risk for heart issues – including clots, inflammation, and arrhythmias – a risk that persists even in relatively healthy people long after the illness has passed.’
© 2022 Stephanie Desmon & Ziyad Al-Aly / Johns Hopkins.
📖 (23 Feb 2023 ~ Journal of Neurology) ‘Patterns of acute ischemic stroke and intracranial hemorrhage in patients with COVID-19’. ➲
‘220 patients (49.8%) presented with respiratory symptoms, and 167 (37.8%) presented with neurological symptoms first.
Acute ischemic stroke (AIS) was detected in 70 (15.8%), and intracranial hemorrhage (IH) in 48 (10.9%) patients.
There was no association between AIS or IH and the severity of COVID-19.’
© 2023 Jensen-Kondering & Maurer et al / Journal of Neurology.
📖 (22 Jun 2023 ~ British Heart Foundation) ‘Nearly 100,000 more deaths involving heart conditions and stroke than usual since pandemic began’. ➲
‘Nearly 100,000 more people with cardiovascular disease than expected have died since the start of the pandemic in England.’
© 2023 British Heart Foundation.
📖 (4 Aug 2022 ~ Fortune) ‘Blood clots, heart problems, kidney failure: COVID creates a higher risk for rare pediatric health problems, new CDC study finds’. ➲
‘Children and teens who’ve had COVID are at greater risk for blood clots, heart problems, kidney failure, and Type 1 diabetes.’
© 2022 Erin Prater / Fortune.
📖 (2 Aug 2022 ~ Nature: News) ‘Heart disease after COVID: what the data say’. ➲
‘But even people who had not been hospitalized had increased risks of many conditions, ranging from an 8% increase in the rate of heart attacks to a 247% increase in the rate of heart inflammation.’
© 2022 Saima May Sidik / Nature: News.
📖 (7 Feb 2022 ~ Nature: Medicine) ‘Long-term cardiovascular outcomes of COVID-19’. ➲
‘Our results provide evidence that the risk and 1-year burden of cardiovascular disease in survivors of acute COVID-19 are substantial.’
© 2022 Yan Xie, Evan Xu, Benjamin Bowe & Ziyad Al-Aly / Nature: Medicine.
📖 (3 Mar 2023 ~ JAMA) ‘One-Year Adverse Outcomes Among US Adults With Post-COVID-19 Condition vs Those Without COVID-19 in a Large Commercial Insurance Database’. ➲
‘Results from this study indicated a statistically significant increased risk for a range of cardiovascular conditions as well as mortality.
While these risks were heightened for individuals who experienced a more severe acute episode of COVID-19 (ie. requiring hospitalization), it is essential to note that most individuals (72.5%) in the cohort did not experience hospitalization during the acute phase.
Many of these conditions will have lasting effects on quality of life.’
© 2023 DeVries & Shambhu et al / JAMA.
📖 (10 Feb 2022 ~ Nature: News) ‘Heart-disease risk soars after COVID – even with a mild case’. ➲
‘Heart-disease risk soars after COVID – even with a mild case: Massive study shows a long-term, substantial rise in risk of cardiovascular disease, including heart attack and stroke, after a SARS-CoV-2 infection.’
© 2022 Saima May Sidik / Nature: News.
📖 (24 Apr 2020 ~ The Washington Post / The Seattle Times) ‘Healthy people in their 30s and 40s, barely sick with COVID-19, are dying from strokes’. ➲
‘[Physician-researcher] Mocco, who has spent his career studying strokes and how to treat them, said he was “completely shocked” by the analysis.
He noted the link between COVID-19 and stroke “is one of the clearest and most profound correlations I’ve come across”.’
© 2020 Ariana Eunjung Cha / The Washington Post / The Seattle Times.
📖 (1 Nov 2021 ~ Harvard Medical School) ‘COVID-19 diagnosis raises risk of heart attack, stroke’. ➲
‘In the week after a COVID-19 diagnosis, the risk of a first heart attack increased by three to eight times.
The risk of a first stroke caused by a blood clot multiplied by three to six times.’
© 2021 Julie Corliss / Harvard Medical School.
📖 (31 Jan 2022 ~ University of Utah) ‘COVID-19 Increasing Stroke Risks in People of All Ages’. ➲
‘“We are definitely seeing a huge increase in younger stroke survivors who are post-COVID diagnosis,” Kinzinger says. “We know that vascular complications go along with COVID infections, which can lead to strokes and other cardiovascular issues.”’
© 2022 University of Utah.
📖 (10 Jan 2023 ~ Frontiers in Cardiovascular Medicine) ‘SARS-CoV-2 infection predicts larger infarct volume in patients with acute ischemic stroke’. ➲
‘Hypercoagulation and microthrombosis represent the typical features of COVID-19 caused by SARS-CoV-2 [infection].
The pathogenic mechanisms are not yet fully understood, but multiple pathways are involved, including endothelialitis*.
The relative risk for the occurrence of AIS [acute ischemic stroke] is 3- to 4-fold greater compared to that of non-infected hospitalized historical or contemporary control cohorts, and since the outcome of AIS patients with COVID-19 appear worse both in terms of initial stroke severity and functional outcome/mortality, this subgroup of infected patients deserves particular attention.’
* Note: Endothelialitis refers to the inflammation of the endothelial cells that line the endothelium, which is the inner lining of blood vessels.
© 2023 De Michele & Lorenzano et al / Frontiers in Cardiovascular Medicine.
📖 (30 Aug 2022 ~ The Financial Times) ‘The growing evidence that Covid-19 is leaving people sicker: The potential impact on heart and brain disease poses challenges to healthcare systems globally’. ➲
‘Researchers found that rates of many conditions, such as heart failure and stroke, were substantially higher in people who had recovered from Covid than in similar people who had not been infected.
Although the evidence is still coming into focus, it is already becoming clearer to clinicians and health leaders in medical systems around the world that they are coping with a higher burden of disease in the population.’
© 2022 Sarah Neville / The Financial Times.
📖 (4 Jun 2020 ~ Science Daily) ‘Coronavirus linked to stroke in otherwise healthy young people’. ➲
‘Young patients with no risk factors for stroke may have an increased risk if they have contracted COVID-19, whether or not they are showing symptoms of the disease.’
© 2020 Thomas Jefferson University / Science Daily.
📖 (24 Aug 2022 ~ Nature: News) ‘Could tiny blood clots cause long COVID’s puzzling symptoms?’ ➲
‘Researchers already know that people with COVID-19, especially severe disease, are more likely to develop clots. The virus can infect cells lining the body’s 100,000 kilometres of blood vessels, causing inflammation and damage that triggers clotting.’
© 2022 Cassandra Willyard / Nature: News.
📖 (Sep 2020 ~ The Lancet) ‘COVID-19 related stroke in young individuals’. ➲
‘We believe that, in otherwise healthy, young patients who present with stroke during the pandemic, the diagnosis of COVID-19 should be thoroughly investigated.’
© 2020 Johanna T Fifia & J Mocco / The Lancet: Neurology.
📖 (9 Feb 2022 ~ Science) ‘COVID-19 takes serious toll on heart health – a full year after recovery: Giant study shows striking rise in long-term heart and vessel disease’. ➲
‘The results are “stunning… worse than I expected, for sure,” says Eric Topol, a cardiologist at Scripps Research.
“All of these are very serious disorders… If anybody ever thought that COVID was like the ‘flu, this should be one of the most powerful data sets to point out that it’s not.”
“In the post-COVID era, COVID might become the highest risk factor for cardiovascular outcomes,” greater than well-documented risks such as smoking and obesity, says Larisa Tereshchenko, a cardiologist and biostatistician at the Cleveland Clinic.’
© 2022 Meredith Wadman / Science.
📖 (15 Sep 2023 ~ The American Journal of Cardiology) ‘Risk of Cardiovascular Events After COVID-19’. ➲
‘Either symptomatic or asymptomatic SARS-CoV-2 infection is associated with increased risk of late cardiovascular outcomes and has causal effect on all-cause mortality in a late post-COVID-19 period.’
© 2023 Tereshchenko & Bishop et al / The American Journal of Cardiology.
📖 (21 Feb 2022 ~ The Washington Post) ‘Five months post-covid, Nicole Murphy’s heart rate is still doing strange things’. ➲
‘Indeed, as the months since their infections have turned into years, people who initially had mild or even some asymptomatic coronavirus cases are pouring into cardiology practices across the country.’
© 2022 Ariana Eunjung Cha / The Washington Post.
📖 (1 Apr 2022 ~ Weill Cornell Medicine) ‘Are COVID-19-Linked Arrhythmias Caused by Viral Damage to the Heart’s Pacemaker Cells?’ ➲
‘The SARS-CoV-2 virus can infect specialized pacemaker cells that maintain the heart’s rhythmic beat, setting off a self-destruction process within the cells, according to a pre-clinical study.’
© 2022 Weill Cornell Medicine.
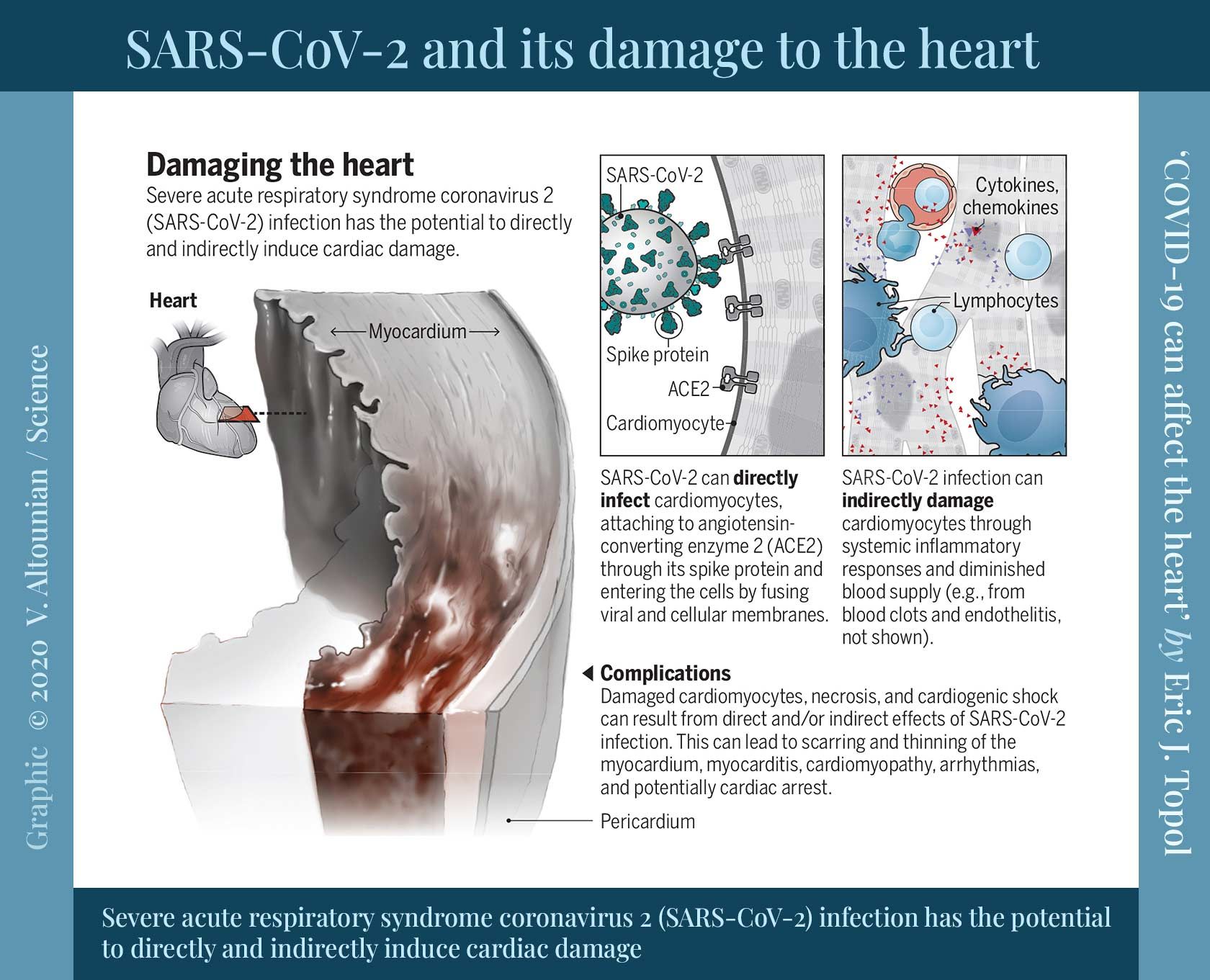
📖 (23 Sep 2020 ~ Science) ‘COVID-19 can affect the heart: COVID-19 has a spectrum of potential heart manifestations with diverse mechanisms’. ➲
‘Collectively, these young, healthy individuals had mild COVID-19 but were subsequently found to have unsuspected cardiac pathology.’
© 2020 Eric J. Topol / Science.
📖 (Updated: 12 May 2023 ~ The British Heart Foundation) ‘Is coronavirus a disease of the blood vessels?’ ➲
‘By triggering endothelial damage, coronavirus infection may cause abnormal blood clotting, ‘leaky’ vessels and reduced blood flow. These endothelial effects could have consequences from head to toe.
In the lungs, these effects can cause clots and fluid to accumulate, which means the lungs are less able to get oxygen into the body.
In the brain, damage to endothelial cells in the blood-brain barrier could lead to inflammation.
In the limbs, reduced blood flow could lead to “Covid toe”.’
© 2023 The British Heart Foundation.
📖 (17 Oct 2022 ~ Nature: Acta Pharmacologica Sinica) ‘Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies’. ➲
‘It has been increasingly appreciated that COVID-19 is not only an infectious disease involving the lung; but also, a vascular disease affecting extrapulmonary organs.
Endothelial dysfunction-induced endotheliitis/endothelialitis/endotheliopathy following SARS-CoV-2 infection arises from a plethora of physiopathological mechanisms, including both direct mechanism or indirect mechanisms.’
© 2022 Xu, Ilyas & Weng / Nature: Acta Pharmacologica Sinica.
📖 (7 Feb 2022 ~ Massachusetts General Hospital / Science Daily) ‘COVID-19-associated strokes link to higher disability and death risk, study finds’. ➲
‘The data suggest that patients from less affluent areas may have been at greater risk for serious complications such as stroke because of their inability to carry out protective measures such as social distancing or working at home.’
© 2022 Massachusetts General Hospital / Science Daily.
📖 (15 Mar 2022 ~ Thomas Jefferson University) ‘International Multi-Center Study Confirms Stroke Risk in Younger Healthier COVID-19 Patients’. ➲
‘The stroke patients who tested positive for COVID-19 were often younger, had multiple large vessels blocked, and often had worse outcomes than our usual patients. This international study confirms those early, alarming observations.’
© 2022 Thomas Jefferson University.
📖 (16 Aug 2022 ~ Penn Medicine News) ‘People with Severe COVID-19 Face Increased Risk of Life-Threatening Blood Clots’. ➲
‘“Our research found this association both before and during vaccine availability, showing that the risk was not stemming from vaccination,” said Vincent Lo Re III, MD, MSCE, an associate professor of Infectious Diseases and Epidemiology at Penn.’
© 2022 Penn Medicine News.
📖 (6 Apr 2022 ~ The Guardian) ‘Covid linked to 33-fold increase in risk of potentially fatal blood clot: Infection with virus also associated with fivefold increase in risk of deep vein thrombosis, data suggests’. ➲
‘Although the risks were highest in patients with more severe illness, even those with mild Covid had a threefold increased risk of DVT and a sevenfold increased risk of pulmonary embolism.’
© 2022 Linda Geddes / The Guardian.
📖 (Updated: 16 Dec 2024 ~ The British Heart Foundation) ‘How does Covid-19 affect your heart?’ ➲
‘Research suggests that Covid-19 can increase the risk of heart and circulatory conditions, like heart attack and stroke, after infection.
Damage can lead to abnormal blood clotting, ‘leaky’ blood vessels and reduced blood flow. Covid-19 can also lead to inflammation of the heart muscle (myocarditis) and heart lining (pericarditis).
Some research suggests that Covid-19 could be linked to an increased risk of developing high blood pressure (hypertension). This is a serious condition, as high blood pressure raises your chances of having a heart attack or stroke.’
© 2024 The British Heart Foundation.
📖 (8 Sep 2022 ~ MedPage Today) ‘Inflammation a Culprit in Long COVID Heart Problems: Prospective study illuminates mechanisms of lingering heart issues after mild COVID-19’. ➲
‘Their study included only patients without previously known cardiac conditions, comorbidities, or abnormal lung function tests at the baseline assessment and who had not been hospitalized for acute COVID-19 at any point.’
© 2022 Crystal Phend / MedPage Today.
📖 (5 Sept 2022 ~ Nature: Medicine) ‘Long-term cardiac pathology in individuals with mild initial COVID-19 illness’. ➲
‘Baseline measurements from 346 individuals with COVID-19 (52% females) were obtained at a median of 109 days after infection, when 73% of participants reported cardiac symptoms, such as exertional dyspnea (62%), palpitations (28%), atypical chest pain (27%) and syncope (3%).
At follow-up (329 days after infection), 57% of participants had persistent cardiac symptoms.
Female gender and diffuse myocardial involvement on baseline imaging independently predicted the presence of cardiac symptoms at follow-up.’
© 2022 Puntmann & Martin et al / Nature: Medicine.
📖 (26 Sep 2022 ~ Nature: Medicine) ‘Lingering cardiac involvement in previously well people after mild COVID-19’. ➲
‘In this article, the authors report a novel association between heart inflammation and prolonged shortness of breath, palpitations and chest pain, which are common after mild COVID-19.’
© 2022 Research Briefing ~ Nature: Medicine.
📖 (19 Sep 2022 ~ American Heart Association) ‘Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales’. ➲
‘Associations between COVID-19 and thrombotic events did not vary markedly by age or sex, but were greater in people of Black or Asian race than those of White race, and in people without than with a history of vascular events.’
© 2022 Knight & Walker et al / American Heart Association.
📖 (28 Sep 2022 ~ Springer Nature) ‘Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: association with autonomic dysregulation’. ➲
‘Subjects in the post-acute COVID-19 phase present autonomic dysregulation… and impaired heart rate variability, which are associated with central aortic stiffness, ventricular-arterial impairment, and left ventricular function impairment.’
© 2022 Oikonomou & Stamatios Lampsas et al / Springer Nature: Heart and Vessels.
📖 (29 Sep 2022 ~ Brisbane Times) ‘Unlike flu, COVID-19 attacks DNA in the heart: new research’. ➲
‘Rather than extreme inflammation which they had expected to find, inflammation signals had been suppressed in the hearts of the COVID-19 patients, while markers for DNA damage and repair were much higher than in people who had died from the ’flu.
The way the DNA damage presented was similar to the way chronic diseases such as diabetes or even cancer presented, with the tissues of the heart putting out DNA damage signals.’
❦ Study: ‘Transcriptomic profiling of cardiac tissues from SARS-CoV-2 patients identifies DNA damage’ by Kulasinghe & Liu et al / Immunology (15 Sep 2022) ➲
© 2022 Stuart Layt / Brisbane Times.
📖 (6 Oct 2022 ~ Fortune) ‘Strokes, heart attacks, sudden deaths: Does America understand the long-term risks of catching COVID?’ ➲
‘Significantly, the risk of some of these complications is stronger in younger adults.
As the virus becomes more immune-evasive, our arsenal is shrinking, not expanding, despite what the CDC [the USA’s Centers for Disease Control] and political leaders may claim.’
© 2022 Carolyn Barber / Fortune.
📖 (21 Apr 2021 ~ American Heart Association) ‘SARS-CoV-2 and Stroke Characteristics: A Report From the Multinational COVID-19 Stroke Study Group’. ➲
‘36% of the AIS [Arterial Ischemic Stroke] patients in our study were less than 55 years of age, and 46% were less than 65 years of age.
These proportions are considerably higher than the population-based reports before the pandemic (12.9%–20.7%).’
© 2021 Shahjouei & Tsivgoulis et al / American Heart Association.
📖 (4 Aug 2021 ~ The Journal of Applied Laboratory Medicine) ‘COVID-19: A Serious Vascular Disease with Primary Symptoms of a Respiratory Ailment’. ➲
‘[During SARS-CoV-2 infection,] endothelial cells encompassing the intima [the innermost layer] in arteries, capillaries, veins and the endocardium bind the viral particles via their ACE2 receptors, and consequently become severely inflamed, damaged, and/or destroyed.’
© 2021 Michael Kalafatis / The Journal of Applied Laboratory Medicine.
📖 (24 Oct 2022 ~ Medical Xpress) ‘COVID-19 surges linked to spike in heart attacks’. ➲
‘The spikes in heart attack deaths have tracked with surges of COVID-19 infection – even during the presumed less-severe Omicron phase of the pandemic.
Furthermore, the data showed the increase was most significant among individuals ages 25–44.’
© 2022 Cedars-Sinai Medical Center / Medical Xpress.
📖 (29 Sep 2022 ~ Journal of Medical Virology) ‘Excess risk for acute myocardial infarction mortality during the COVID-19 pandemic’. ➲
‘The trend of mortality suggests that age and sex disparities have persisted even through the recent Omicron surge, with excess AMI*-associated mortality being most pronounced in younger-aged adults.’
* Note: AMI = Acute myocardial infarction, or heart attack.
© 2022 Yeo & Wang et al / Journal of Medical Virology.
📖 (24 Oct 2022 ~ Bloomberg UK) ‘Covid-19 Tied to Higher Risk of Deadly Blood Clots in Large Study’. ➲
‘Non-hospitalized Covid patients were 2.7 times more likely to develop dangerous clots called venous thromboembolisms and were more than 10 times more likely to die than individuals who avoided the disease.’
© 2022 Jason Gale / Bloomberg UK.
📖 (25 Oct 2022 ~ CNBC) ‘People who caught mild Covid had increased risk of blood clots, British study finds’. ➲
‘[P]atients hospitalized with Covid were 28 times more likely to develop blood clots, 22 times more likely to suffer heart failure, and 17 times more likely to have a stroke, according to the study.’
© 2022 Spencer Kimball / CNBC.
📖 (24 Oct 2022 ~ British Medical Journal: Heart) ‘Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank’. ➲
‘Overall, available evidence supports a distinct mechanistic role for COVID-19 in driving higher VTE* rates which occurs across disease severities and extends beyond the early post-infection phase.’
* Note: Venous thromboembolism (VTE) = refers to a blood clot that starts in a vein.
© 2022 Raisi-Estabragh & Salih et al / British Medical Journal: Heart.
📖 (27 Apr 2021 ~ JACC: Basic to Translational Science) ‘SARS-CoV-2 Infects Human Engineered Heart Tissues and Models COVID-19 Myocarditis’. ➲
‘This study provides evidence that cardiomyocytes are a target of SARS-CoV-2 in the human heart, and support the conclusion that SARS-CoV-2 infection of cardiomyocytes and resultant… inflammation contribute to the cardiac manifestations of COVID-19.’
© 2021 Bailey & Dmytrenko et al / JACC: Basic to Translational Science.
📖 (19 Nov 2022 ~ Pediatric Neurology) ‘SARS-CoV-2 Infection and Increased Risk for Pediatric Stroke’. ➲
‘COVID-19 infection can lead to a delayed hyperinflammatory response…
Based on epidemiologic data, we hypothesize that there is likely a delay of at least one month from the timing of initial COVID-19 infection to the development of stroke.
Our study suggests that prior COVID-19 infection, but not acute infection, is correlated with a risk for stroke in the pediatric population.
The risk for stroke appears to be distinct from the risk for [Multisystem Inflammatory Syndrome in Children/MIS-C].’
© 2022 Vielleux & Swartwood / Pediatric Neurology.
📖 (17 Oct 2022 ~ News Medical Life Sciences) ‘Asymptomatic COVID could potentially cause more complications in trauma patients’. ➲
‘Compared with similar trauma patients who tested negative for COVID, positive asymptomatic COVID trauma patients had higher rates of myocardial infarction and cardiac arrest (3.2% vs 0.9%).’
© 2022 American College of Surgeons / News Medical Life Sciences.
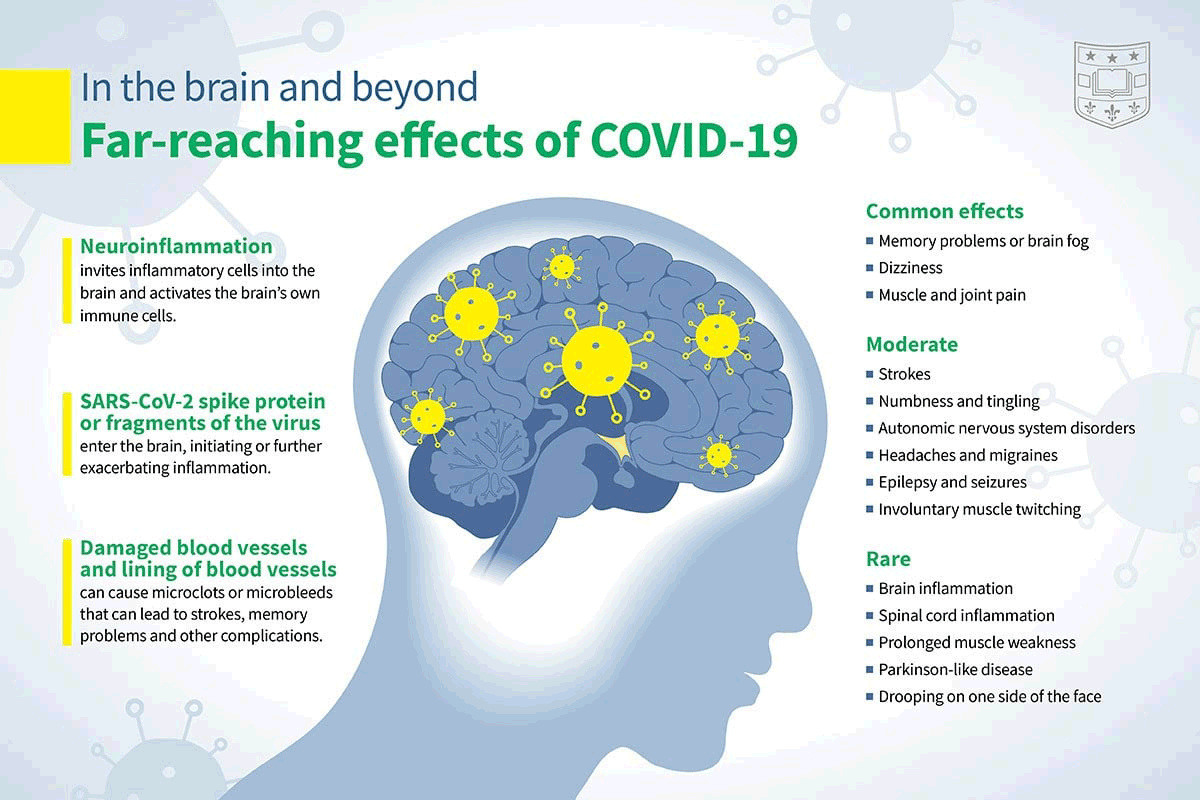
📖 (20 Jan 2022 ~ Science) ‘Nervous system consequences of COVID-19’. ➲
‘[High-field MRI] examination of brain tissue demonstrates microvascular damage in structures plausibly related to neurologic manifestations of COVID-19, consistent with endothelial activation and widespread vascular injury observed in other organs.’
© 2022 Serena Spudich & Avindra Nath / Science.
📖 (12 Nov 2022 ~ Springer Nature) ‘Inflammation and vascular remodeling in COVID-19 hearts’. ➲
‘We conclude that cardiac involvement in COVID-19 is an angiocentric macrophage-driven inflammatory process, distinct from classical anti-viral inflammatory responses, and substantially underappreciated by conventional histopathologic analysis.
A systematic follow-up of non-fatal COVID-19 and long-COVID cases [would help detect] post-acute cardiac symptoms and fibrotic remodeling also in mild cases of disease due to the irreversible alterations of the cardiac vasculature.’
© 2022 Werlein & Ackermann et al / Springer Nature: Angiogenesis.
📖 (8 Jul 2022 ~ American Heart Association) ‘Thromboinflammation: From Atherosclerosis to COVID-19’. ➲
‘The activating interplay of thrombosis [blood clotting] and inflammation (thromboinflammation) has been established as a major underlying pathway, driving not only cardiovascular disease but also autoimmune disease and most recently, COVID-19.
Endothelial dysfunction and thromboinflammation have emerged early on as the key pathogenic mechanisms driving COVID-19 pathology…
COVID-19–specific coagulopathy is epitomized by elevated levels of [several glycoproteins involved in blood clotting].’
© 2022 D. Wagner & L. Heger / American Heart Association: Arteriosclerosis, Thrombosis, and Vascular Biology.
📖 (26 Dec 2022 ~ Nature: Communications) ‘Transcriptional reprogramming from innate immune functions to a pro-thrombotic signature by monocytes in COVID-19’. ➲
‘These results [reflect] hypercoagulability and acquired coagulopathies in patients with COVID-19, and suggest that monocytes from moderate COVID-19 patients upregulate a pro-thrombotic gene expression signature upon secondary SARS–CoV–2 sensing.’
© 2022 Allison & Burnham et al / Nature: Communications.
📖 (2 Dec 2022 ~ Time) ‘Tiny Blood Clots May Be to Blame for Long COVID Symptoms, Some Researchers Say’. ➲
‘[Stellenbosch University Physiological Sciences’ Chair] posits that lingering viral remnants may damage the cells that line the blood vessels, prompting the formation of inflammation and microclots, which could in turn make the immune system attack itself.’
© 2022 Jamie Ducharme / Time.
📖 (23 Aug 2021 ~ Springer Nature) ‘Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin’. ➲
‘Results presented in the current paper point to a significant failure in the fibrinolytic process [enzymatic breakdown of intravascular fibrin in blood clots] during COVID-19 and also in patients with lingering Long COVID/PASC symptoms.’
© 2021 Etheresia Pretorius & Mare Vlok / Springer Nature: Cardiovascular Diabetology.
📖 (26 Dec 2022 ~ Journal of Clinical Medicine) ‘Mid- and Long-Term Atrio-Ventricular Functional Changes in Children after Recovery from COVID-19’. ➲
‘60% of children who recovered from asymptomatic or mildly symptomatic COVID-19 still exhibit mild subclinical systolic cardiac impairment after an average follow-up of 148 ± 68 days from disease onset.’
© 2022 Sabatino & Di Chiara et al / Journal of Clinical Medicine.
📖 (13 Jan 2023 ~ Nature Reviews: Microbiology) ‘Long COVID: major findings, mechanisms and recommendations’. ➲
‘Cardiac MRI studies revealed cardiac impairment in 78% of 100 individuals who had a prior COVID-19 episode (investigated an average of 71 days after infection) and in 58% of participants with long COVID (studied 12 months after infection), reinforcing the durability of cardiac abnormalities.’
© 2023 Hannah E. Davis, Lisa McCorkell, Julia Moore Vogel & Eric J. Topol / Nature Reviews: Microbiology.
📖 (21 Jan 2023 ~ The Sydney Morning Herald) ‘Fatal heart attacks have surged in Australia. Here’s why’. ➲
‘“COVID-19 infection worsens pre-existing heart conditions, and increases the risk of developing more than 20 heart conditions including heart attack, blood clots, heart failure and stroke,” [Dr. Amanda Buttery, Heart Foundation of Australia] said.’
© 2023 Aisha Dow / The Sydney Morning Herald.
📖 (28 Nov 2022 ~ The Medical Journal of Australia) ‘Associations between COVID‐19 and hospitalisation with respiratory and non‐respiratory conditions: a record linkage study’. ➲
‘The incidence of hospitalisation with cerebral infarction [ischemic stroke] was twice as high after COVID‐19 onset as during the baseline period. Other investigators have estimated the risk of stroke to be 2–13 times as high for people with COVID‐19.
The pathophysiology of cerebrovascular involvement is unclear, but may be related to substantial endothelial disruption in highly vascularised organs such as the brain.’
© 2022 Rowe & Leder et al / The Medical Journal of Australia.
📖 (19 Jan 2023 ~ European Society of Cardiology) ‘COVID-19 patients retain elevated risk of death for at least 18 months after infection’. ➲
‘COVID-19 is associated with higher risks of cardiovascular disease and death in the short- and long-term, according to a study in nearly 160,000 participants.
Compared to uninfected individuals, the likelihood of COVID-19 patients dying was up to 81 times higher in the first three weeks of infection and remained five times higher up to 18 months later.’
© 2023 Sophia Antipolis / European Society of Cardiology.
📖 (25 Jan 2023 ~ SciTech Daily / American Heart Association) ‘COVID Toll: Big Jump in Cardiovascular-Related Deaths Reported by American Heart Association’. ➲
‘COVID-19 has both direct and indirect impacts on cardiovascular health. As we learned, the virus is associated with new clotting and inflammation.’
© 2023 American Heart Association / SciTech Daily.
📖 (13 Jan 2023 ~ Nature Reviews: Microbiology) ‘Long COVID: major findings, mechanisms and recommendations’. ➲
‘The narrative that COVID-19 had only respiratory sequelae led to a delayed realization of the neurological, cardiovascular and other multisystem impacts of COVID-19.’
© 2023 Hannah E. Davis, Lisa McCorkell, Julia Moore Vogel & Eric J. Topol / Nature Reviews: Microbiology.
📖 (2 Feb 2023 ~ Nature Communications) ‘High-depth sequencing characterization of viral dynamics across tissues in fatal COVID-19 reveals compartmentalized infection’. ➲
‘This lends credence to the hypothesis that direct infection may lead to the heart-related sequelae observed in some COVID-19 patients.
SARS-CoV-2 may establish a viral reservoir in these anatomical sites, leading to on-going tissue-specific pathology’
© 2023 Normandin & Rudy et al / Nature Communications.
📖 (15 Feb 2023 ~ Journal of the American Heart Association) ‘Collagen‐Specific HSP47+ Myofibroblasts and CD163+ Macrophages Identify Profibrotic Phenotypes in Deceased Hearts With SARS‐CoV‐2 Infections’. ➲
‘Cardiac fibrosis complicates SARS-CoV-2 infections and has been linked to arrhythmic complications in survivors.
[Post-infection cardiac scarring may lead to] enhanced arrhythmogenesis, and even sudden cardiac death. As such, COVID‐19–induced cardiac fibrosis may have critical long‐term public health implications for healthcare systems and policy makers.’
© 2023 Puzyrenko & Jacobs et al / Journal of the American Heart Association.
📖 (8 Mar 2023 ~ Journal of Clinical Medicine) ‘Long-Term Adverse Effects of Mild COVID-19 Disease on Arterial Stiffness, and Systemic and Central Hemodynamics: A Pre-Post Study’. ➲
‘The finding that the longer the period from COVID-19 infection the worse the vascular impairment was surprising, as we expected inflammation burden associated with COVID-19 to decrease with time.’
© 2023 Podrug & Koren et al / Journal of Clinical Medicine.
📖 (19 May 2023 ~ EurekAlert) ‘International COVID-19 Registry uncovers increased incidence of clotting in heart attack patients with COVID-19’. ➲
‘COVID-19 is a pro-inflammatory, clot-forming disease and we now see its effect in the coronary arteries…
These new insights point to the need for clinicians to be meticulous with blood thinning strategies, early interventions and patient follow-up.’
© 2023 Society for Cardiovascular Angiography & Interventions / EurekAlert.
📖 (10 Aug 2023 ~ Associated Press) ‘COVID-19 took a toll on heart health and doctors are still grappling with how to help’. ➲
‘Long COVID patients were about twice as likely to seek care for cardiovascular problems like blood clots, abnormal heartbeats or stroke in the year after infection, compared to similar patients who’d avoided COVID-19. — U.S. insurance database analysis.’
© 2023 Lauran Neergaard / Associated Press.
📖 (21 Aug 2023 ~ American Heart Association / Medical Xpress) ‘Analysis finds COVID-19 may trigger new-onset high blood pressure’. ➲
‘An analysis of electronic medical records for more than 45,000 people found that COVID-19 infection was significantly associated with the development of high blood pressure, according to new research published in Hypertension.’
© 2023 American Heart Association / Medical Xpress.
📖 (28 Sep 2023 ~ National Institutes of Health) ‘SARS-CoV-2 infects coronary arteries, increases plaque inflammation’. ➲
‘SARS-CoV-2, the virus that causes COVID-19, can directly infect the arteries of the heart and cause the fatty plaque inside arteries to become highly inflamed, increasing the risk of heart attack and stroke, according to a study funded by the NIH.’
© 2023 National Institutes of Health (NIH / USA).
📖 (28 Sep 2023 ~ Nature: Cardiovascular Research) ‘SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels’. ➲
‘Our data establish that SARS-CoV-2 infects coronary vessels, inducing plaque inflammation that could trigger acute cardiovascular complications and increase the long-term cardiovascular risk.’
© 2023 Eberhardt & Noval et al / Nature: Cardiovascular Research.
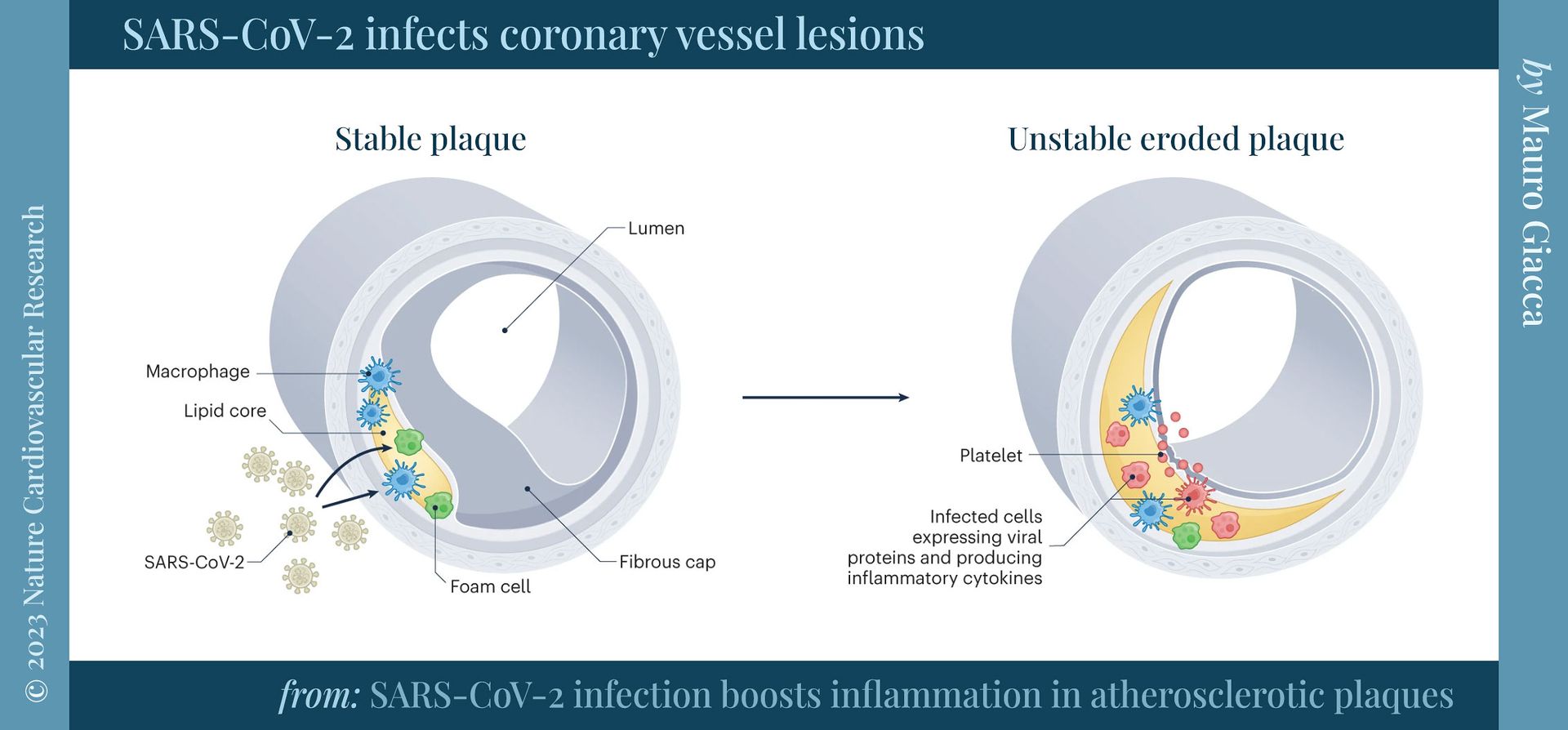
📖 (18 Oct 2023 ~ Nature: Cardiovascular Research) ‘SARS-CoV-2 infection boosts inflammation in atherosclerotic plaques’. ➲
‘A post-mortem study reported that severe vascular injury, including microthrombosis, was nine times more prevalent in COVID-19 lungs than inpatients with influenza.’
© 2023 Mauro Giacca / Nature: Cardiovascular Research.
📖 (18 Oct 2023 ~ Nature: Cardiovascular Research) ‘SARS-CoV-2 infection boosts inflammation in atherosclerotic plaques (Fig. 1: SARS-CoV-2 infects coronary vessel lesions)’. ➲
‘SARS-CoV-2 induces a strong pro-atherogenic inflammatory response… This pro-inflammatory response may contribute to the instability of atherosclerotic plaques and the increased incidence of ischemic cardiovascular complications in patients.’
© 2023 Mauro Giacca / Nature: Cardiovascular Research.
📖 (9 Nov 2023 ~ European Heart Journal) ‘Prior COVID-19 infection is associated with persistent and higher thrombus burden in acute coronary syndromes’. ➲
‘Our findings suggest that COVID-19 infection may lead to persistent endothelial dysfunction and hypercoagulability, portending increased severity of coronary artery ectasia and coronary thrombosis even after recovery from the initial infection.’
© 2023 To-Dang & Zuckerman / European Heart Journal.
📖 (18 May 2022 ~ American Medical Association) ‘3 tips for doctors trying to manage long COVID’s cardiac symptoms’. ➲
‘As many as 10% to 30% may develop long COVID following SARS-CoV-2 infection. In these individuals, symptoms such as chest pain, shortness of breath, and palpitations draw attention to the cardiovascular system.
Patients with long COVID may experience other cardiac symptoms such as elevated blood pressure, tachycardia out of proportion to that expected for effort, and drops in oxygen saturation.
There is also a lack of data to help guide diagnosis, treatment and prognosis.
That is why a systematic approach to assist in the evaluation and management of long COVID is needed, especially since the evidence base is likely to evolve over time.
Patient-centered models of care are needed to address long COVID.
This requires
co-ordination
by
multi-disciplinary
teams
that include
primary care physicians,
sub-specialists
such as
pulmonologists,
cardiologists, and
neurologists, along with
social workers,
psychologists, and
physical therapists.’
© 2022 Sara Berg / American Medical Association.
More... Cardiovascular System