📖 Immune damage in Long Covid
❦ ‘Acute infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cause a respiratory illness that can be associated with systemic immune cell activation and inflammation, widespread multi-organ dysfunction, and thrombosis.
Not everyone fully recovers from COVID-19, leading to Long Covid, the treatment of which is a major unmet clinical need.
Long Covid can affect people of all ages, follows severe as well as mild disease, and involves multiple organs.
Patients with Long Covid display signs of immune dysfunction and exhaustion, persistent immune cell activation, and autoimmune antibody production, which are also pathological features of acute COVID-19.
The complement system is crucial for innate immune defense by effecting lytic destruction of invading micro-organisms, but when uncontrolled, it causes cell and vascular damage.
The complement cascade is activated by antigen–antibody complexes in the classical pathways or in the lectin pathway by multimeric proteins (lectins) that recognize specific carbohydrate structures, which are also found on the SARS-CoV-2 spike protein that facilitates host cell entry.
Both pathways may contribute to the pronounced complement activation in acute COVID-19.
Long Covid symptoms include a postexertional exhaustion reminiscent of other post-viral illnesses, such as myalgic encephalomyelitis (ME) – chronic fatigue syndrome (MECFS) with suspected latent viral reactivation.
Antibody titer changes in Long Covid patients indicate an association of fatigue with reactivation of latent Epstein-Barr virus (EBV) infections, and Cervia-Hasler et al found that the severity of Long Covid symptoms is associated with cytomegalovirus (CMV) reactivation.
A better understanding of the connections between viral reactivation, persistent interferon signaling, and autoimmune pathologies promises to yield new insights into the thromboinflammation associated with Long Covid.
Although therapeutic interventions with coagulation and complement inhibitors in acute COVID-19 produced mixed results, the pathological features specific for Long Covid suggest potential interventions for clinical testing.
Microclots are also observed in ME-CFS patients, indicating crucial interactions between complement, vWF, and coagulation-mediated fibrin formation in post-viral syndromes.
A better definition of these interactions in preclinical and clinical settings will be crucial for the translation of new therapeutic concepts in chronic thromboinflammatory diseases.’
❂
📖 (18 Jan 2024 ~ Science) Immune damage in Long Covid ➤
© 2024 Wolfram Ruf / Science.
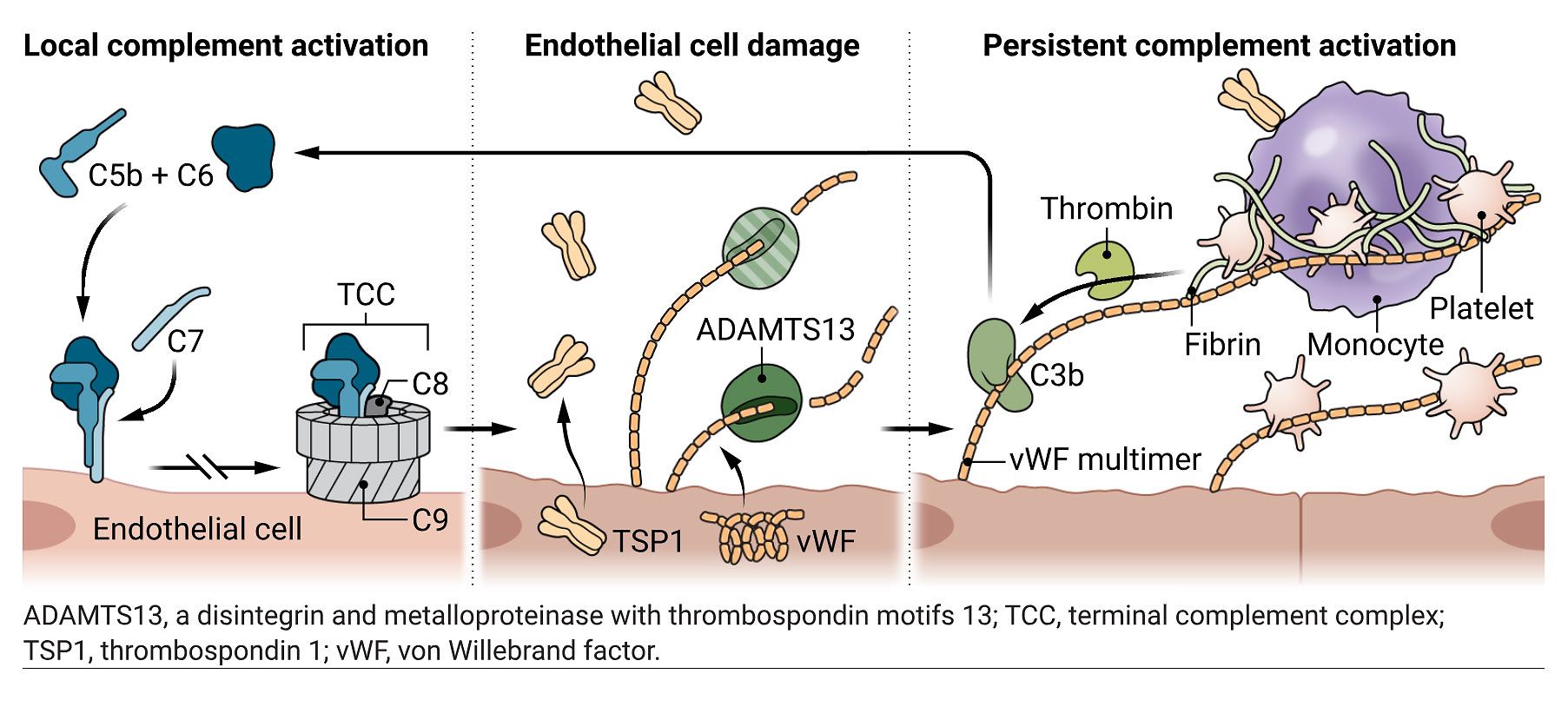
Complement-coagulation cross-talk at the endothelial interface.
During acute COVID-19, the complement and coagulation systems can become activated, and remain locally activated in various tissues in Long Covid patients.
Endothelial cell damage by membrane insertion of the complement C5b-C7 complex results in vWF and TSP1 release.
This is highly prothrombotic because the resulting vWF multimers recruit platelets and promote thrombin generation, and TSP1 promotes monocyte interactions with platelets.
Additionally, reduced ADAMTS13 in Long Covid patients promotes the accumulation of ultralarge vWF multimers, which induces C3b binding and stimulates the alternative pathway of complement activation.
Together, these feedback loops can maintain local complement activation and inflammation, as well as generating microclots that could underlie some of the features of Long Covid.
📖 (18 Jan 2024 ~ Science) Immune damage in Long Covid ➤
© 2024 Science.